Abstract
Because the variable ability of the antibody constant (Fc) domain to recruit innate immune effector cells and complement is a major factor in antibody activity in vivo, convenient means of assessing these binding interactions is of high relevance to the development of enhanced antibody therapeutics, and to understanding the protective or pathogenic antibody response to infection, vaccination, and self. Here, we describe a highly parallel microsphere assay to rapidly assess the ability of antibodies to bind to a suite of antibody receptors. Fc and glycan binding proteins such as FcγR and lectins were conjugated to coded microspheres and the ability of antibodies to interact with these receptors was quantified. We demonstrate qualitative and quantitative assessment of binding preferences and affinities across IgG subclasses, Fc domain point mutants, and antibodies with variant glycosylation. This method can serve as a rapid proxy for biophysical methods that require substantial sample quantities, high-end instrumentation, and serial analysis across multiple binding interactions, thereby offering a useful means to characterize monoclonal antibodies, clinical antibody samples, and antibody mimics, or alternatively, to investigate the binding preferences of candidate Fc receptors.
Introduction
Research and development of clinically relevant antibody therapeutics, as well as an increasingly refined understanding of the humoral response to infection and vaccination, has demonstrated the critical importance of antibodies across a range of disease states. In vivo, effector function, that is, the ability of an antibody to interact with antibody receptors expressed solubly in plasma, on the surface of innate immune effector cells, or even intracellularly following internalization of immune complexes, is an important aspect of antibody activity. As such, mechanistic understanding of how antibodies can link antigen recognition to potent biological effect through the spectrum of Ig receptors is of critical therapeutic relevance.
The binding affinity of an IgG for Fc receptors (FcR) can be modulated by IgG subclass,Citation1 Fc domain glycosylation,Citation2 avidity driven by immune complex formation,Citation3,Citation4 IgG multimerization,Citation5 variant disulfide bond formation,Citation6 or via amino acid point mutations identified by recombinant protein engineering methodsCitation7 or those present naturally among GM allotypes.Citation8,Citation9 The resulting combinatorial diversity in antibody characteristics is complemented by diversity among antibody receptors, which even among classical FcγR vary in subclass binding preferences, glycan sensitivity, cellular distribution and expression level, and can lead to outcomes ranging from immunosuppression to secretion of lytic factors.
For protein therapeutics, rational modulation of these collective effector functions via subclass and isotype choice, glycoengineering, amino acid point mutations, or via entirely novel binding domains promises to allow specific effector functions to be alternatively enhanced or ablated as desired.Citation10,Citation11 Likewise, some of these modifications are available to B cells, with longstanding evidence that IgG subclass selection is highly regulated, and increasing evidence that the immune system is able to actively tune antibody activity based on variant glycosylation.Citation12-Citation15 Collectively, these natural mechanisms offer a path for similar rational induction of antibody responses with specific functional profiles via vaccination.Citation16
Furthermore, beyond relatively well-characterized FcγR and complement proteins, a growing number of diverse and structurally unrelated Fc-binding proteins have been identified, ranging from the pH-sensitive neonatal Fc receptorCitation17 to C-type lectins such as dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN),Citation18 FcR-Like receptors,Citation19,Citation20 mannose-binding lectin 2 (MBL2),Citation21 TRIM21,Citation22 macrophage mannose receptor (MMR),Citation23 and Dectin-1.Citation24 Probing the recognition properties of these and other FcR for engineered and naturally-produced IgG represents an important avenue to enhance our understanding of their potential role in antibody activity in vivo. Lastly, understanding the FcγR binding dynamics of other ligands of interest, such as pentraxins (pattern recognition molecules that are considered innate antibodies),Citation25 or pathogen-secreted molecules that can interfere with FcγR function,Citation26 or the development of therapeutic inhibitors of FcγR may also be crucial to providing high-resolution understanding of the role of antibodies and antibody receptors in immunity and recombinant antibody therapies.
Thus, high-throughput means to characterize either the ability of therapeutic proteins of interest to interact with these receptors or the ability of candidate Fc receptors to interact with different antibody species could be of high value. To this end, we report the development of a multiplexed coded microsphere assay to simultaneously assess IgG Fc – Fc receptor interactions at high throughput with minimal sample requirements. We demonstrate qualitative and quantitative assessment of binding preferences and affinities across IgG subclasses, Fc domain amino acid point mutants identified by protein engineering methods, and antibodies with variant glycosylation. This characterization is performed across classical FcγR, complement proteins, several recently described FcR, and glycan-recognizing lectin proteins. The highly parallel assessment of antibody:FcR interactions enabled by the microsphere array described here provides a rapid proxy for biophysical methods requiring substantial sample quantities, high-end instrumentation, and serial analysis across multiple binding interactions.
Results
Multiplexed assessment of IgG subclass specificity for FcγR and complement
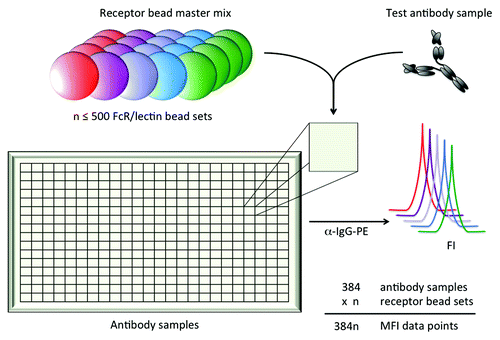
As a means to rapidly assess either the FcR binding preferences of antibody samples of interest or the antibody binding preferences of FcR of interest, we explored use of multiplexed fluorescently-coded magnetic microspheres. To this end, FcR were covalently conjugated to coded microspheres through primary amines. Individually coded FcR bead sets were combined, incubated in test antibody samples, and antibody bound to each FcR was quantified using fluorescence of a detection reagent in a third channel as depicted in .
Figure 1. Schematic depiction of multiplexed assessment of IgG interactions with FcR and lectins. Up to 500 bead sets, each coupled with an FcR or lectin of interest, can be combined into one well containing an antibody sample of interest. Bound antibody is then quantified for each unique receptor bead set using the fluorescent intensity (FI) of a PE-conjugated detection reagent as measured on a Luminex reader.
To evaluate whether the well-characterized binding preferences of FcγR and C1q for different IgG subclasses could be recapitulated in the multiplexed microsphere array format, monomeric IgG1, IgG2, and IgG3 from serum and myeloma-derived IgG4 were serially diluted and incubated with fluorescently-coded FcγR and C1q-conjugated microspheres. presents the background-subtracted median fluorescent intensities (MFI) for each IgG subclass over concentrations ranging from 2 μM to 100 pM across the complete set of human FcγR allotypic variants and C1q. We detected relative relationships between IgG subclasses, receptors and receptor allotypes consistent with previous studies.Citation27-Citation29 As expected, the high affinity FcγRI receptor exhibited signal at significantly lower antibody concentrations than the lower affinity FcγR. Among FcγRII variants, affinity was observed to decrease as follows: FcγRIIa H131 > FcγRIIa R131 ≈FcγRIIb/c. Similarly, the FcγRIIIa V158 allotype exhibited a higher signal than the FcγRIIIa F158 allotype at equivalent antibody concentrations, and FcγRIIIb variants exhibited the lowest (data not shown). Among the IgG subclasses, IgG3 exhibited a higher signal than other subclasses for several FcγR, including FcγRI, the low affinity F158 variant of FcγRIIIa, across all three FcγRIIIb allotypes (data not shown), as well as for C1q. The enhanced ability of IgG2 to bind the FcγRIIa H131 allotype over the R131 allotype was observed; otherwise, binding of monomeric IgG2 to FcγR was generally low. Direct comparisons with data regarding IgG4 is hampered by the limited availability of plasma-derived IgG4. As a substitute, myeloma-derived IgG4 was used, and was notable in that it generally bound weakly to the FcγRs and C1q. While this material may not exhibit the extensive diversity of glycan structures present in serum-derived, polyclonal IgG, it demonstrates the expected binding profile. Lastly, the dynamic range for each FcγR bead set spanned 2–4 orders of magnitude.
Figure 2. Multiplexed assessment of IgG subclass specificity of FcγR and complement. Extracellular domain allotypes of human FcγR and C1q were simultaneously screened for the ability to bind to fractionated serum IgG1, IgG2, IgG3, and myeloma-derived IgG4, revealing diverse recognition patterns both among IgG subclasses and between FcγR allotypic variants. The median fluorescent intensity (MFI) of antibody bound to each microsphere set was determined across a wide concentration range. Dilutions were performed in duplicate and error bars represent one standard deviation.
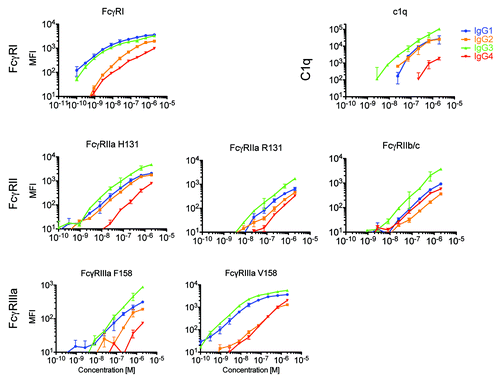
Thus, dose-response curves and single concentration MFIs qualitatively match expectations for relationships both among subclasses, where IgG1 and IgG3 exhibit the highest affinity and IgG2 and IgG4 weaker affinity to FcγR, and among receptors, where high and low affinity receptors were easily resolved, as were differences among receptor allotypes. It is worth noting that while MFI signals can be used to compare antibody subclass binding for each receptor, the MFI values for each receptor are not directly comparable because there may be differences in conjugation efficiencies or receptor sizes resulting in different levels of FcR captured on each bead, or differences in orientation and accessibility of the Fc binding site among FcR depending on the position of the amine residues used in the conjugation reaction.
Simultaneous testing of multiple Fc receptors poses twin challenges to making accurate measurements of antibody binding. First, depletion of analyte is a potential confounder, particularly given the multiplexed and low volume nature of the assay. This possibility was assessed by calculation: the number of receptors on each bead was approximated by dividing the total bead area by the cross sectional area of a 25 kD receptor estimated using the Stokes equation,Citation30 and the number of antibodies captured from solution was calculated using the Langmuir isotherm. Based on this calculation, even at the lowest test concentration, a negligible fraction of total antibody is expected to be bead-bound. Second, and perhaps more importantly, simultaneous testing of multiple FcγR establishes experimental conditions under which these receptors must compete for antibody binding given their overlapping epitopes. For a homogeneous antibody population, the calculation above indicates that competition would have a limited impact. However, for a heterogeneous population, as is expected due to variation in glycosylation, an influence of competition cannot be excluded, though it could be either mathematically estimated or experimentally assessed.
Multiplexed prediction of equilibrium binding affinity
To further investigate the ability of the multiplexed array to more quantitatively capture binding affinity differences, a set of previously designed Fc domain point mutants was selected, as described in .Citation31,Citation32 Fc mutations associated with enhanced binding to multiple or individual FcγR were constructed for VRC-01, a broadly neutralizing HIV-specific antibody, and trastuzumab, a HER2-specific therapeutic antibody. Kinetic parameters, including the rate constants kon and koff and the equilibrium constant KD for a subset of these mutants were measured by surface plasmon resonance (SPR; Table S1), and were in good agreement with previous studies.Citation31,Citation32
Table 1. Fc domain point mutant panel
As an example of the qualitative relationships observed between equilibrium KD and array MFI, we compared wildtype Fc with SD/IE and SE/HF/ST/IE/GA mutants, which have significantly enhanced affinity to FcγRIIIa and FcγRIIa, respectively. (left) presents the MFI observed for wildtype and mutant trastuzumab at a single concentration (667 nM) when bound to microspheres conjugated with either FcγRIIa H131 (top) or FcγRIIIa F158 (bottom). The array MFI values demonstrate good qualitative agreement with binding affinity (1/KD) as determined by SPR (right) for both receptors, and clearly recapitulate the higher binding affinity of the SD/IE mutant (green) for FcγRIIIa, and SE/HF/ST/IE/GA (red) for FcγRIIa.
Figure 3. Multiplexed prediction of equilibrium binding affinity. Qualitative (A) and quantitative (B,C) relationships between multiplex receptor array signal intensity (MFI) and the equilibrium binding affinities (KD) of the Fc domain point mutant panel. A. Qualitative comparison of multiplex array MFI (left) and affinity (right) as measured by SPR for wildtype (WT) and a pair of trastuzumab backbone Fc domain mutants (SD/IE and SE/HF/ST/IE/GA) with enhanced affinity for FcγRIIa (top) or FcγRIIIa (bottom). B. Quantitative comparison of array MFI vs. published equilibrium binding affinities for C1q for the complement relevant Fc mutants (run in duplicate) on the VRC-01 backbone. C. Quantitative comparison of the equilibrium binding affinity derived from the microsphere array (KD ≈EC50) vs. published values across multiple FcγR for the Fc mutant panel (run in duplicate) on the VRC-01 backbone.
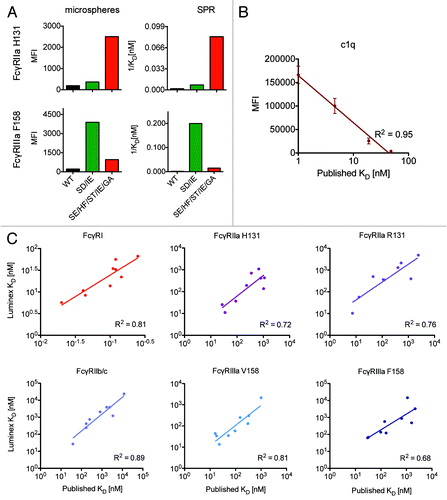
To extend this qualitative analysis, MFI values observed at an antibody concentration of 1200 nM for Fc variants with enhanced binding to C1q are plotted against published equilibrium KD in , showing excellent quantitative agreement (R2 = 0.95). Moreover, for binding isotherms where saturation was achieved, the Luminex titration curves could be used to derive effective binding affinities (KD ≈EC50). These effective KD’s are plotted against published equilibrium KD values for each of the VRC-01 point mutants across FcγR in . Again, quantitative agreement was good, with an average R2 = 0.78. Significantly, for each mutant, data regarding binding across the complete set of FcγR and complement was captured simultaneously in a single experimental well using an antibody sample volume of only 40 μl per well. Additionally, the affinities of the interactions tested spanned over 4 orders of magnitude, from sub nM to μM, establishing a wide dynamic range and convenient experimental conditions. Similar results were observed for mutants of both Fv domain specificities. There is an upper limit of sensitivity for monovalent interactions due to fast off rates where, with a dissociation constant greater than 5 μM, considerable antibody dissociates during the wash steps, reducing fluorescent signal during the detection readout. For high affinity interactions with a dissociation constant less than 0.1 nM, one should extend the incubation time appropriately to ensure that low concentration test samples reach equilibrium.
An additional experiment was performed to evaluate the ability to detect the pH-specific interaction with the FcRn receptor. Wild type VRC-01 at 2000 nM was incubated at pH 6.0 and pH 7.2 with FcRn and the panel of Fc gamma receptors including FcγRI, IIa, IIb/c and IIIa The relative MFI of pH 6.0 vs. pH 7.2 increased 250 to 500% for FcRn while the Fcγ receptors changed by only 35% on average, indicating the assay is capable of assessing binding to FcRn (data not shown).
Probing glycan dependence
Because the interaction of the IgG Fc domain with FcγR is known to be highly dependent on the glycan at position N297, we next determined the ability of the multiplexed array to capture the effects of gross and fine differences in antibody glycosylation. Pooled plasma IgG from HIV-positive donors (HIVIG) was treated with the N-glycosidase PNGaseF, and native or N-deglycosylated HIVIG samples were tested for the ability to bind FcγR at 267 nM. A glycan-insensitive anti-human IgG bead set was used as a negative control. presents the MFI of HIVIG and PNGase-treated HIVIG relative to native HIVIG. Removal of N-linked glycans dramatically reduced binding to all FcγR, while leaving recognition by the anti-human IgG control bead relatively intact. Despite N-deglycosylation, some residual binding of FcγRI is apparent. Discrepant data regarding the glycan-sensitivity of this receptor exists,Citation33,Citation34 with recent suggestions that observations of FcγRI-binding activity of deglycosylated IgG may be due to avid interactions.Citation35 This possibility cannot be excluded by our results as the HIVIG IgG samples were not subjected to size exclusion chromatography (SEC) following deglycosylation.
Figure 4. Probing glycan dependence of polyclonal antibody samples. A,B: FcγR (A) or lectin (B) conjugated beads were incubated with 267 nM of native IgG pooled from HIV positive donors (HIVIG) or HIVIG treated with PNGaseF to remove N-linked glycans. For comparability of fluorescent signals across multiple bead sets, HIVIG binding to each bead set was assigned an MFI of 1 and binding of de-glycosylated HIVIG to each bead type was plotted on this relative scale. An anti-human IgG capture bead (IgG) was used a positive control to ensure de-glycosylation had not compromised antibody structure. The mean of 3 replicates is plotted and error bars denote the %CV. C,D: In a separate experiment, native and de-glycosylated HIVIG were mixed in varying proportions before being incubated with FcγR (C) or lectin (D) beads in order to determine the dose-dependence of glycosylation status. Relative MFI scale established with respect to native HIVIG for each bead set, as in A,B.
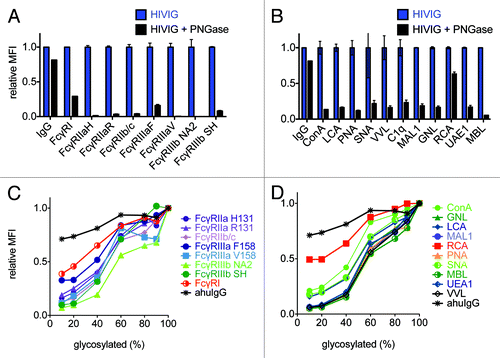
Furthermore, as the specific N297 glycoform is known to affect antibody function, we investigated whether lectins, which are sugar-binding proteins with defined glycan specificities but similarly low affinities and relatively low signal-to-noise in array methods,Citation36 could be adapted to microsphere analysis. While FcγR ligation is clearly an important functional marker, the ability to associate FcγR binding preferences to specific glycan species or relative compositions would provide the ability to link functional differences with structural alterations. Thus, as a potential alternative to high performance liquid chromatography or mass spectrometry, we determined whether lectin-conjugated microspheres could be used to profile antibody glycosylation status. presents binding of native and N-deglycosylated HIVIG across a set of lectins. Residual binding of PNGaseF-treated HIVIG is generally higher on the lectin beads relative to FcγR beads, possibly due to the presence of non N-linked glycans or a minor population of IgG with residual, uncleaved N-linked glycan. Lastly, dose-responsive FcγR and lectin recognition of variably glycosylated IgG is demonstrated in . Here, native and PNGaseF-treated IgG samples were mixed at different ratios, and a direct relationship between fractional glycosylation and array MFI is observed.
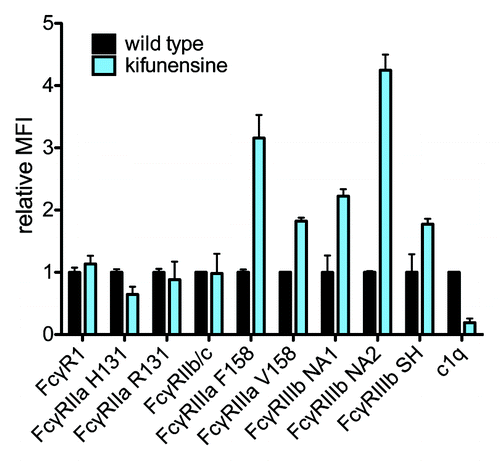
As complete deglycosylation is a gross modification, we investigated whether finer alterations known to affect antibody activity, and potentially more reflective of the natural diversity of serum IgG glycan species, could be detected. Thus, VRC-01 was glycoengineered by expression in the presence of a class I α-mannosidase inhibitor, kifunensine, which results in production of antibodies with oligomannose glycans lacking fucose, galactose, and sialic acid,Citation37,Citation38 as compared with wildtype VRC-01, which is primarily fucosylated, and partially galactosylated, and sialylated. compares the MFI vs. antibody concentration from 0.1 nM to 1 μM for kifunensine-treated vs. wild type VRC-01 using a panel of 4 lectins. It was observed that ConA exhibited a preference for oligomannose, kifunensine-treated VRC-01 (5A), in contrast to AAL (5B), SNA (5C), and RCA (5D), which recognize fucose, sialic acid, and galactose, respectively, and preferred wild type VRC-01. These observations are consistent with the lectins’ known preferences. compares FcγR and C1q binding of kifunensine-treated vs. wild type VRC-01. The nonfucosylated, high oligomannose VRC-01 produced under mannosidase inhibition exhibits increased binding to all FcγRIII receptors, and reduced binding to C1q, consistent with SPR measurements (Table S1) and previous studies.Citation39,Citation40
Figure 5. Probing glycan dependence of glycoengineered monoclonal antibodies. A-D: VRC-01 cultured with and without kifunensine was incubated with a panel of lectin-coupled microspheres to evaluate the assay’s ability to probe fine antibody glycoform composition. The median fluorescent intensity (MFI) of antibody bound to lectin-coupled microspheres specific for mannose (ConA, A), fucose (AAL, B), sialic acid (SNA, C), or galactose (RCA, D) was determined across a wide concentration range. Dilutions were performed in duplicate and error bars represent one standard deviation.
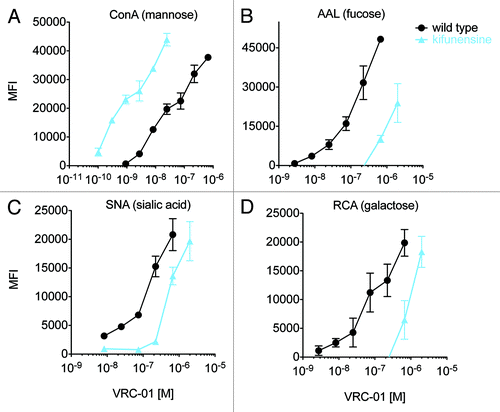
Figure 6. Probing glycan dependence of FcR. The relative FcγR and complement binding of wild type and glycoengineered (kifunensine-treated) VRC-01 having nonfucosylated and oligomannose glycans. Samples were tested at a single concentration in triplicate, and error bars represent one standard deviation. The mean of binding of wildtype VRC-01 across replicates was assigned a relative MFI of 1 for each receptor bead set.
Facile identification of non-classical FcR binding preferences
As an alternative to profiling differences in the effector function of IgG samples, the microsphere format could be utilized to investigate the binding preferences of putative FcR. Accordingly, we examined the subclass and glycan specificities of several non-classical Fc-binding receptors, including Dectin-2, FcR-like 5 (FcRL5), MMR (MRC1 or CD206), MBL2, and DC-SIGN. While the binding preferences of a number of these receptors are relatively uncharacterized, there have been reports of glycan preferences among the C-type lectins used here; for example, DC-SIGN, Dectin-2, MBL2, and MMR are known to recognize mannosylated proteins.Citation41-Citation44 Thus, the kifunensine-cultured VRC-01, rich in oligomannose, was analyzed for binding to these receptors. Receptor binding to the glycoengineered variant and the nonglycosylated point mutant N297Q relative to wild type VRC-01 at a fixed concentration (667 nM) is shown in . Binding of oligomannose VRC-01 was increased over 100-fold for DC-SIGN, Dectin-2 and MBL2, and increased 20-fold for MMR, in agreement with their known mannose specificity. Notably, with respect to a number of these receptors, including DC-SIGN, relative binding of non-glycosylated VRC-01 was equivalent to wildtype, consistent with relatively low specificity for the Fc glycans prevalent in recombinantly-produced antibody. Low and equivalent overall signals for both wildtype and non-glycosylated IgG also indicated low specificity for the Fc protein backbone (data not shown). By contrast, non-glycosylated VRC-01 exhibited a lesser (70%) reduction of binding to FcRL5 relative to wildtype, similar to that observed for FcγR (FcγRIIIb NA2 shown for comparison). These results are consistent with recent publications.Citation45,Citation46 Interestingly, oligomannose-sensitive binding of FcRL5 has not been previously observed.
Figure 7. Glycan preference and buffer dependence of non-classical FcR. A. The relative ability of VRC-01 produced by untreated (wildtype) or kifunensine-treated cells and a non-glycosylated mutant (N297Q), to bind to DC-SIGN, Dectin-2 FcRL5, MBL2 and MMR was assessed. The MFI observed for each of 3 replicates relative to the mean MFI observed for wildtype VRC-01 is presented. B. The calcium-dependence and ability of mannan (+ Man) to compete for binding of VRC-01 produced with kifunensine treated cells against a panel of FcR is presented.
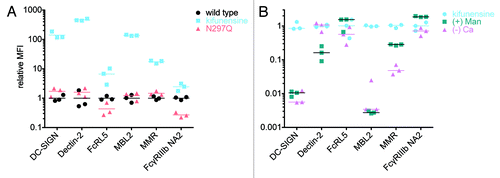
A follow-up study was performed to evaluate the calcium dependence of these receptors, as well as the ability of 200 μg/ml mannan to inhibit binding (). The lack of calcium and presence of mannan reverted binding of oligomannose IgG to DC-SIGN and MBL2 back to wild type levels (100-fold decrease), indicating that the interaction with these receptors may be largely due to glycan binding and relatively non-specific to IgG Fc, consistent with previous findings for DC-SIGN.Citation46,Citation47 The lack of calcium did not affect binding to Dectin-2, while the presence of mannan reduced binding signal by 80%. MMR binding was decreased 70% in the absence of calcium and the presence of mannan reduced signal 20-fold. In contrast, neither Dectin-2 nor MMR receptor signals were completely reduced under these buffer conditions, consistent with potential stabilization with IgG Fc by protein:protein interactions. As a negative control, FcγRIIIb NA2 was observed to be neither highly calcium dependent nor competed with free mannan. Binding of serum IgG1, IgG2 and IgG3 to these receptors was analyzed and yielded similar results (data not shown).
Effect of antibody valency on FcγR binding
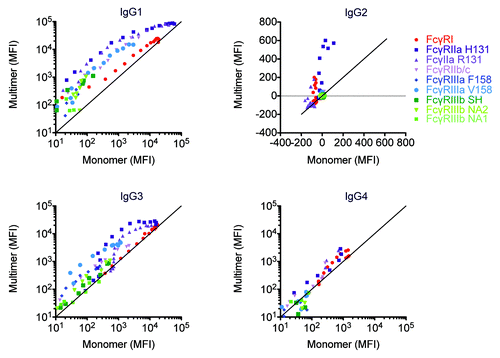
Lastly, numerous previous studies have shown that IgG aggregates or multimers induced via heat treatment, Fab ligation, or antigen-driven formation of immune complexes, can dramatically increase the binding to FcγR. Accordingly, in this study, SEC was utilized to ensure the IgG samples were monomeric. However, the higher molecular weight species were collected separately and a comparison of FcγR binding was made between the monomeric and multimeric species. presents a scatterplot of the binding signals (MFI) observed for equivalent concentrations of mono- and multimeric myeloma-derived IgG1, IgG2, and IgG4 or plasma-derived IgG3 fractions across the complete set of human FcγR. Among the FcγR, FcγRI appears to be relatively insensitive to valency, with data points generally lying on the y = x line, though some evidence of avidity-driven enhancement of binding for IgG1 at low concentrations is apparent. Given the high affinity of FcγRI, this result is expected. By contrast, antibody multimers exhibit significantly higher MFI than monomers at equivalent concentrations among the low affinity FcR. Among the IgG subclasses, avid interactions dramatically affected IgG1 and IgG3 interactions with FcγR, but exhibited a lesser effect on the lower affinity IgG2 and IgG4 subclasses. Significant enhancement of avid IgG2 interactions was only observed for the FcγRIIa H131 allotype, and of avid IgG4 interactions for the FcγRII variants. In general, these observations are consistent with the effects expected given the affinity of each subclass for each FcγR.
Figure 8. Avidity differentially modulates FcγR:antibody ligation among IgG subclasses and FcγR. Myeloma derived IgG1, IgG2, and IgG4 and pooled serum derived IgG3 were separated into monomeric and multimeric fractions by SEC. Across a range of matched concentrations, the ability of IgG monomer and multimer samples to bind FcγR was assessed.
Discussion
The importance of antibody effector function is well recognized by both the therapeutic antibody and vaccine research communities. Because of the extensive diversity and pleiotropic activities of Fc receptors, methods capable of characterizing receptor-antibody binding in parallel across multiple receptors hold promise in enhancing understanding of the global impact of Fc domain variation, whether among designed or natural variants.
Here, we demonstrate multiplexed assessment of Fc domain binding interactions capable of resolving qualitative and quantitative differences in recognition among IgG subclasses, Fc domain point mutants, and antibodies with variant glycosylation across a range of Fc receptors. As such, this method could be applied to rapidly screen binding relationships across FcR from diverse species in order to better translate results from animal studies, or to assess efforts aimed at endowing alternative scaffolds with highly tailored effector function. Alternatively, the low sample requirements, but comprehensive data output, motivates adaptation of this method to evaluate the FcR binding profile of clinical antibody samples such as antigen-specific or total plasma IgG—potentially furthering our understanding of the protective or pathogenic antibody response to infection, vaccination, and self.
Beyond the canonical FcγR, we explored the IgG binding preferences of a number of receptors that have more recently been cited to bind IgG. These include a set of mannose-sensitive proteins such as DC-SIGN, Dectin-2, MBL2, and MMR,Citation41-Citation44 each of which exhibited strikingly enhanced binding to oligomannosylated IgG. Interestingly, binding to DC-SIGN and MBL2 was almost completely inhibited in the presence of mannan, and these receptors appeared to bind wildtype VRC-01 and a non-glycosylated N297Q mutant equivalently poorly. The IgG binding capacity and preferences of DC-SIGN in particular have been controversial.Citation18,Citation48-Citation50 This controversy may be partially explained by the heterogeneity of antibody glycosylation. Given binding enhancements of 100-fold for some glycoforms, even relatively minor species could dramatically affect the overall binding activity of a sample of interest. Similarly, clear differences in the binding of the IgG subclasses depending on source (recombinant, myeloma, or serum-derived) were observed here. Each source bears its own set of caveats. Commercially-available serum-derived subclasses are likely composed of multiple GM allotypes, and have the advantage of bearing a natural distribution of glycoforms; however, they may be contaminated with as much as 5% of IgG1. Similarly, a myeloma-derived preparation may or may not be broadly representative of a given subclass, and recombinant antibodies clearly exhibit different glycosylation patterns compared with plasma IgG. Furthermore, differences between monoclonal and polyclonal Fab domains, particularly in the context of receptors recognizing glycans, could also affect results.
A critical quality attribute to control in all evaluations of antibody Fc binding activity is the monomeric state of the IgG samples. If samples are not monomeric, calculated affinities may be more representative of the extent of sample aggregation than affinity.Citation51 Indeed, the multiplex assay could be used to compare the binding characteristics of mono- and multimeric antibodies, exposing striking differences in the ability of antibodies of different subclasses to exploit avidity. These data are consistent with previous studies that have found that multimerization of antibodies with undetectable FcR binding when tested as monomers can result in FcR-dependent cellular activation, and that immune complex size, subclass composition, and glycosylation each play a role in this activity.Citation4 Such results emphasize the fact that antibodies do not typically act in isolation in vivo; instead, they act in the context of immune complexes, which may exhibit emergent properties reflective of their nature as antibody ensembles.
Overall, we demonstrated that assessment of Fc domain binding characteristics can be adapted to microsphere format with high sensitivity (sub nM to μM), rapid turnaround (less than 4 h), multiplexed throughput (up to 500 interactions), low receptor requirements (5 μg for hundreds of assays) and low sample requirements (10 μg or less), providing substantial advantages over traditional binding assays on cells, plate-based ELISA, or SPR. Relative to printed arrays, microspheres have advantages in terms of ease of customization and adaptation, good long-term stability, gentle chemistry and avoidance of prolonged storage in unusual buffers or in semi-dry states. Thus, this method can serve as a rapid proxy for biophysical methods that require significant sample quantities, high-end instrumentation, and serial analysis across multiple binding interactions, offering a useful means to characterize the Fc receptor binding capacity of monoclonal or clinical antibody samples, or to investigate the binding preferences of candidate Fc receptors.
Materials and Methods
Recombinant protein vector construction and expression
Construction and expression of VRC-01 and trastuzumab
CMV/R mammalian expression vectorsCitation52 for the VRC-01Citation53 IgG1 light and heavy chains were obtained from the NIH AIDS Reagent Program. The trastuzumab variable domain was obtained via gene synthesis and was reformatted as a full-length IgG using an overlap extension PCR strategy relying on primers designed with homology to the CH1 and Cκ domains of VRC-01, followed by digestion and ligation into cut CMV/R vector. Following sequence verification, DNA was isolated via maxi-prep (Qiagen), and used to transfect suspension cultures of human embryonic kidney (HEK) 293F cells grown in Freestyle media (Invitrogen) using 25 kD branched PEI (PolySciences) as previously described.Citation54 Antibodies were transiently expressed for 7 d at 37 °C, 8% CO2. Fc domain amino acid point mutations () were incorporated via quickchange PCR (Stratagene), and expression in the presence 20 μM kifunensine (Tocris) was utilized to produce nonfucosylated, oligomannose Fc glycans.Citation37 Sequences for each construct are available in the Supplemental Information.
Construction and expression of human Fc receptors
The extracellular domains (ECDs) of human Fcγ receptors, including FcγRI, FcγRIIa H131, FcγRIIb/c, FcγRIIIa V158, FcγRIIIB NA2, and nonclassical Fc receptors including DC-SIGN, Dectin-2, MBL2 (Open Biosystems) were PCR amplified using a forward primer incorporating the CMV/R leader sequence and reverse primer including a sortase A cleavage site (LPXTG) and 6-his tag to enable affinity purification and site-specific conjugation and complementary restriction sites to enable ligation into the CMV/R vector. Allotypic variants in receptor ECDs were generated by single or multiple point mutations using the quickchange strategy (Stratagene) for FcγRIIa R131, FcγRIIIa F158, FcγRIIIb NA1, and FcγRIIIB SH. After clone selection and sequence verification, receptors were transiently expressed in HEK293-F cells as described above. Gene insert information is available in the Supplemental Information section. FcRL5, MMR, C1q, and lectins were commercially-sourced (R&D Systems; Vector Labs). FcRn was produced as described previously.Citation55 lists the set of FcR and lectins tested. Complete sequence information is available in the Supplemental Information.
Table 2. Fc receptors and lectins
Antibody and FcR preparation
Seven days after transfection, cells were separated by centrifugation and culture supernatant was 0.2 μm filtered (Steritop Express, Millipore). Antibody production supernatants were loaded onto a Mabselect Protein A column (GE) with a residence time of 1 min and affinity-purified using an AKTA FPLC system (GE). The column was washed with 5 column volumes (CV) of PBS (Teknova) and eluted with 5 CV of 100 mM glycine pH 3.0. The peaks were neutralized with a 1.6% v/v spike of 1 M Tris-Cl at pH 10.5. The neutralized peaks were 0.2 μm filtered (Steriflip Millipore), concentrated with a 10 kD membrane (Amicon Ultra-15, EMD-Millipore) and loaded over a SEC column (HiPrep 16/60 Sephacryl S-200 HR, GE), operating under the manufacturers recommendations with PBS to remove aggregates. IgG1, IgG2, IgG4 (Athens Research) and IgG3 (Sigma Aldrich) from myeloma plasma and serum IgG1, IgG2 and IgG3 from healthy donors (Athens Research) were also purified using a Sephacryl S-200 column to remove aggregates. Pooled IgG from HIV-positive donors (NIH AIDS Reagent Resource Program) was deglycosylated as follows: dilute IgG (0.1 mg/ml in 20 mM Tris pH 8.2) was digested with 5 units of PNGase F (New England Biolabs) per 100 μg IgG for 1 h at 37 °C with end-over-end mixing. Enzyme was deactivated by 5 min treatment at 100 °C, prior to extensive buffer exchange.
Fc receptors were purified via immobilized metal affinity chromatography (IMAC). Filtered supernatants were spiked with concentrated solutions to bring the final load to 500 mM NaCl, 20 mM sodium phosphate and 20 mM imidazole. The material was loaded onto nickel charged Sepharose 4 Fast Flow column (GE) with a residence time of 1 min. The column was washed with 10 CVs of 500 mM NaCl, 20 mM sodium phosphate and 20 mM imidazole pH 7.5 followed by isocratic elution with 250 mM imidazole. The peaks were 0.2 μm filtered (Steriflip, EMD-Millipore), concentrated with a 10 kD or 3 kD membrane (Amicon Ultra-15, EMD-Millipore) and loaded onto a 120 ml Superdex 75 SEC column (GE) operating under the manufacturers recommendations with PBS. FcγRI was >50% aggregated; therefore 3 rounds of SEC were performed to produce monomeric material. Size and purity of all recombinant protein was confirmed by SDS-PAGE.
Multiplexed microsphere assay
Preparation of receptor and lectin-conjugated microspheres
A customized multivariate microsphere assay was developed using a panel of receptors to carboxylated magnetic fluorescent microspheres (MagPlex-C Microspheres, Luminex Corp.) in an adaptation of a previously described method.Citation56 A total of 1 million carboxylated microspheres were covalently coupled to 5 μg receptor using a two-step carbodiimide reaction. lists the Fc receptors and lectins (Vector Labs) used. Microspheres were washed by centrifugation and magnetic separation, then activated by resuspension in 80 μl of 100 mM monobasic sodium phosphate, pH 6.2, followed by the addition of 10 μl of 50 mg/ml N-hydroxysulfosuccinimide (24520, Pierce) in deionized water and 10 μl of 50 mg/ml 1-ethyl-3-[3 dimethlyaminopropyl]carbodiimide-HCl (77149, Pierce) in deionized water. This reaction mixture was mixed end-over-end on an inverter for 20 min at room temperature. Activated microspheres were then washed three times in 150 μl of PBS, resuspended in 100 μl of PBS, and incubated with 5 μg of Fc receptor, in a final volume of 500 μl of 50 mM MES pH 6.0, on an inverter for 2 h at room temperature. Finally, coupled microspheres were washed with 500 μl of PBS and resuspended in 250 μl of PBS-TBN (PBS, 0.1% BSA, 0.02% Tween 20, 0.05% sodium azide, pH 7.4) for blocking free reactive sites. After either 30 min at room temperature or an overnight incubation at 4 °C in PBS-TBN, microspheres were washed with 500 μl PBS to remove blocking buffer and resuspended in 150 μl of PBS-TBN. The coupled microspheres were counted on an automated cell counter (TC10, Biorad) and stored at 4 °C in the dark for 3–12 mo.
Fluorescent signal detection
A master mix of Fc-binding receptor-coupled microspheres was prepared by combining individual bead sets to achieve a final count of 500 microspheres of each specificity in a 10 μl final volume of incubation/wash buffer comprising 20 mM Tris pH 7.5, 150 mM NaCl, 2 mM CaCl2, 0.05% (v/v) Tween-20 or 10 mM Tris pH 7.5, 1mM CaCl2, 1mM Mg, 1 mM Mn, 0.05% Tween-20. Binding inhibition studies were performed with 200 ug/ml mannan (Sigma Aldrich). Black, clear bottom 384-well plates (Greiner Bio One, 781906) were used. Forty μl of test antibody at 1.25× the target concentration was first added to the wells, then 10 μl of the working microsphere master mix (500 microspheres of each type/well) was added. The plate was covered with microplate adhesive film (USA Scientific), and the bottom of the plate was submerged in a sonicator for 15 s and then incubated on an XYZ-plane plate shaker (IKA, MTS 2/4) at room temperature for 2 h. After incubation, the plate was washed five times with 60 μl of buffer using a plate washer (Biotek, 405 Select TS), and 40 μl of anti-human IgG PE-conjugated detection antibody (Southern Biotech) diluted to 0.65 μg/ml was added to each well. The plate was covered, sonicated and incubated for 30 min at room temperature on an XYZ-plane plate shaker. The microspheres were washed five times as before, and resuspended in 35 μl of Luminex Sheath Fluid. The plate was then covered, sonicated and read in a Bio-plex array reader (FlexMap 3D, Bio-Plex Manager 5.0, Bio-Rad). The median fluorescence intensity (MFI) of PE signal was determined for each bead set in each well. Background signal, defined as the average MFI observed for each microsphere set when incubated with detection reagent in the absence of test antibody, was subtracted from the MFI of each sample.
Kinetic Measurements
Kinetic parameters (kon and koff) and calculated affinity (KD = koff / kon) were measured for a subset of antibody Fc variants using SPR. Experiments were performed using the Bio-Rad ProteOnXPR36 instrument. All 6 channels of a ProteOn GLM chip were coupled in the horizontal orientation with goat anti-human F(ab’)2 antibody (109–005–097, Jackson ImmunoResearch), using 10 mM sodium acetate, pH 4.5 buffer. Antibody variants at 15 μg/ml were injected in the vertical direction for 1 min at 25 μl/min for a target loading of 800 response units (RU), which gave an Rmax of ~100 RU for the FcγR. A 5.5-fold dilution series of each Fc gamma receptor was run in the horizontal direction. The sixth channel was reserved as a blank reference with PBST. The horizontal inter-spots (without antibody bound to the coupled anti-F(ab’)2 surface) and the real-time analyte blank were used to double reference the sensorgrams. After each sample, the chip was regenerated with 25 μl of 5% phosphoric acid (injected in both horizontal, then vertical orientations) and reloaded with test antibody. All experiments were performed in PBST at 25 °C. ProteOn Manager Software (Version 3.1.0.6) was used for data collection and analysis.
Additional material
Download Zip (190.7 KB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
These studies were supported by the Collaboration for AIDS Vaccine Discovery (OPP1032817: Leveraging Antibody Effector Function) to G.A. and M.E.A., the Rheumatology Research Foundation Disease Targeted Research Grant to P.A.N. and M.E.A., 1R21AI099435 to P.A.N., and NIH 1R01AI080289 to G.A. The following reagents were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH: CMVR VRC-01 H and L from Dr. John Mascola, and HIVIG from NABI and NHLBI.
References
- Jefferis R, Pound J, Lund J, Goodall M. Effector mechanisms activated by human IgG subclass antibodies: clinical and molecular aspects. Review article. Ann Biol Clin (Paris) 1994; 52:57 - 65; PMID: 8210076
- Jefferis R, Lund J, Pound JD. IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunol Rev 1998; 163:59 - 76; http://dx.doi.org/10.1111/j.1600-065X.1998.tb01188.x; PMID: 9700502
- Bazin R, Lemieux R, Tremblay T, St-Amour I. Tetramolecular immune complexes are more efficient than IVIg to prevent antibody-dependent in vitro and in vivo phagocytosis of blood cells. Br J Haematol 2004; 127:90 - 6; http://dx.doi.org/10.1111/j.1365-2141.2004.05105.x; PMID: 15384982
- Lux A, Yu X, Scanlan CN, Nimmerjahn F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J Immunol 2013; 190:4315 - 23; http://dx.doi.org/10.4049/jimmunol.1200501; PMID: 23509345
- Teeling JL, Jansen-Hendriks T, Kuijpers TW, de Haas M, van de Winkel JG, Hack CE, Bleeker WK. Therapeutic efficacy of intravenous immunoglobulin preparations depends on the immunoglobulin G dimers: studies in experimental immune thrombocytopenia. Blood 2001; 98:1095 - 9; http://dx.doi.org/10.1182/blood.V98.4.1095; PMID: 11493456
- Liu H, May K. Disulfide bond structures of IgG molecules: structural variations, chemical modifications and possible impacts to stability and biological function. MAbs 2012; 4:17 - 23; http://dx.doi.org/10.4161/mabs.4.1.18347; PMID: 22327427
- Lazar GA, Dang W, Karki S, Vafa O, Peng JS, Hyun L, Chan C, Chung HS, Eivazi A, Yoder SC, et al. Engineered antibody Fc variants with enhanced effector function. Proc Natl Acad Sci U S A 2006; 103:4005 - 10; http://dx.doi.org/10.1073/pnas.0508123103; PMID: 16537476
- de Lange GG. Polymorphisms of human immunoglobulins: Gm, Am, Em and Km allotypes. Exp Clin Immunogenet 1989; 6:7 - 17; PMID: 2698222
- Kumpel BM, Wiener E, Urbaniak SJ, Bradley BA. Human monoclonal anti-D antibodies. II. The relationship between IgG subclass, Gm allotype and Fc mediated function. Br J Haematol 1989; 71:415 - 20; http://dx.doi.org/10.1111/j.1365-2141.1989.tb04300.x; PMID: 2539182
- Nimmerjahn F, Ravetch JV. Translating basic mechanisms of IgG effector activity into next generation cancer therapies. Cancer Immun 2012; 12:13; PMID: 22896758
- Jefferis R. Isotype and glycoform selection for antibody therapeutics. Arch Biochem Biophys 2012; 526:159 - 66; http://dx.doi.org/10.1016/j.abb.2012.03.021; PMID: 22465822
- Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, et al. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J Clin Invest 2013; 123:2183 - 92; http://dx.doi.org/10.1172/JCI65708; PMID: 23563315
- Hess C, Winkler A, Lorenz AK, Holecska V, Blanchard V, Eiglmeier S, Schoen AL, Bitterling J, Stoehr AD, Petzold D, et al. T cell-independent B cell activation induces immunosuppressive sialylated IgG antibodies. J Clin Invest 2013; 123:3788 - 96; http://dx.doi.org/10.1172/JCI65938; PMID: 23979161
- Rombouts Y, Ewing E, van de Stadt LA, Selman MH, Trouw LA, Deelder AM, Huizinga TW, Wuhrer M, van Schaardenburg D, Toes RE, et al. Anti-citrullinated protein antibodies acquire a pro-inflammatory Fc glycosylation phenotype prior to the onset of rheumatoid arthritis. Ann Rheum Dis 2013; Forthcoming http://dx.doi.org/10.1136/annrheumdis-2013-203565; PMID: 24106048
- Bondt A, Selman MH, Deelder AM, Hazes JM, Willemsen SP, Wuhrer M, Dolhain RJ. Association between galactosylation of immunoglobulin G and improvement of rheumatoid arthritis during pregnancy is independent of sialylation. J Proteome Res 2013; 12:4522 - 31; http://dx.doi.org/10.1021/pr400589m; PMID: 24016253
- Ackerman ME, Alter G. Opportunities to exploit non-neutralizing HIV-specific antibody activity. Curr HIV Res 2013; 11:365 - 77; http://dx.doi.org/10.2174/1570162X113116660058; PMID: 24191934
- Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity 1994; 1:303 - 15; http://dx.doi.org/10.1016/1074-7613(94)90082-5; PMID: 7889418
- Anthony RM, Wermeling F, Karlsson MC, Ravetch JV. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc Natl Acad Sci U S A 2008; 105:19571 - 8; http://dx.doi.org/10.1073/pnas.0810163105; PMID: 19036920
- Wilson TJ, Fuchs A, Colonna M. Cutting edge: human FcRL4 and FcRL5 are receptors for IgA and IgG. J Immunol 2012; 188:4741 - 5; http://dx.doi.org/10.4049/jimmunol.1102651; PMID: 22491254
- Wilson TJ, Gilfillan S, Colonna M. Fc receptor-like A associates with intracellular IgG and IgM but is dispensable for antigen-specific immune responses. J Immunol 2010; 185:2960 - 7; http://dx.doi.org/10.4049/jimmunol.1001428; PMID: 20668221
- Arnold JN, Dwek RA, Rudd PM, Sim RB. Mannan binding lectin and its interaction with immunoglobulins in health and in disease. Immunol Lett 2006; 106:103 - 10; http://dx.doi.org/10.1016/j.imlet.2006.05.007; PMID: 16814399
- Keeble AH, Khan Z, Forster A, James LC. TRIM21 is an IgG receptor that is structurally, thermodynamically, and kinetically conserved. Proc Natl Acad Sci U S A 2008; 105:6045 - 50; http://dx.doi.org/10.1073/pnas.0800159105; PMID: 18420815
- Dong X, Storkus WJ, Salter RD. Binding and uptake of agalactosyl IgG by mannose receptor on macrophages and dendritic cells. J Immunol 1999; 163:5427 - 34; PMID: 10553068
- Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, et al. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med 2012; 18:1401 - 6; http://dx.doi.org/10.1038/nm.2862; PMID: 22922409
- Lu J, Marjon KD, Mold C, Du Clos TW, Sun PD. Pentraxins and Fc receptors. Immunol Rev 2012; 250:230 - 8; http://dx.doi.org/10.1111/j.1600-065X.2012.01162.x; PMID: 23046133
- Stemerding AM, Köhl J, Pandey MK, Kuipers A, Leusen JH, Boross P, Nederend M, Vidarsson G, Weersink AY, van de Winkel JG, et al. Staphylococcus aureus formyl peptide receptor-like 1 inhibitor (FLIPr) and its homologue FLIPr-like are potent FcγR antagonists that inhibit IgG-mediated effector functions. J Immunol 2013; 191:353 - 62; http://dx.doi.org/10.4049/jimmunol.1203243; PMID: 23740955
- Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, Daëron M. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716 - 25; http://dx.doi.org/10.1182/blood-2008-09-179754; PMID: 19018092
- Warncke M, Calzascia T, Coulot M, Balke N, Touil R, Kolbinger F, Heusser C. Different adaptations of IgG effector function in human and nonhuman primates and implications for therapeutic antibody treatment. J Immunol 2012; 188:4405 - 11; http://dx.doi.org/10.4049/jimmunol.1200090; PMID: 22461693
- Brüggemann M, Williams GT, Bindon CI, Clark MR, Walker MR, Jefferis R, Waldmann H, Neuberger MS. Comparison of the effector functions of human immunoglobulins using a matched set of chimeric antibodies. J Exp Med 1987; 166:1351 - 61; http://dx.doi.org/10.1084/jem.166.5.1351; PMID: 3500259
- Wasyl Z, Luchter E, Bielański W Jr.. Determination of the effective radius of protein molecules by thin-layer gel filtration. Biochim Biophys Acta 1971; 243:11 - 8; http://dx.doi.org/10.1016/0005-2795(71)90031-6; PMID: 4107733
- Moore GL, Chen H, Karki S, Lazar GA. Engineered Fc variant antibodies with enhanced ability to recruit complement and mediate effector functions. MAbs 2010; 2:181 - 9; http://dx.doi.org/10.4161/mabs.2.2.11158; PMID: 20150767
- Richards JO, Karki S, Lazar GA, Chen H, Dang W, Desjarlais JR. Optimization of antibody binding to FcgammaRIIa enhances macrophage phagocytosis of tumor cells. Mol Cancer Ther 2008; 7:2517 - 27; http://dx.doi.org/10.1158/1535-7163.MCT-08-0201; PMID: 18723496
- Tao MH, Morrison SL. Studies of aglycosylated chimeric mouse-human IgG. Role of carbohydrate in the structure and effector functions mediated by the human IgG constant region. J Immunol 1989; 143:2595 - 601; PMID: 2507634
- Pound JD, Lund J, Jefferis R. Aglycosylated chimaeric human IgG3 can trigger the human phagocyte respiratory burst. Mol Immunol 1993; 30:233 - 41; http://dx.doi.org/10.1016/0161-5890(93)90052-D; PMID: 8381917
- Nesspor TC, Raju TS, Chin CN, Vafa O, Brezski RJ. Avidity confers FcγR binding and immune effector function to aglycosylated immunoglobulin G1. J Mol Recognit 2012; 25:147 - 54; http://dx.doi.org/10.1002/jmr.2155; PMID: 22407978
- Li Y, Tao SC, Bova GS, Liu AY, Chan DW, Zhu H, Zhang H. Detection and verification of glycosylation patterns of glycoproteins from clinical specimens using lectin microarrays and lectin-based immunosorbent assays. Anal Chem 2011; 83:8509 - 16; http://dx.doi.org/10.1021/ac201452f; PMID: 21975078
- Baruah K, Bowden TA, Krishna BA, Dwek RA, Crispin M, Scanlan CN. Selective deactivation of serum IgG: a general strategy for the enhancement of monoclonal antibody receptor interactions. J Mol Biol 2012; 420:1 - 7; http://dx.doi.org/10.1016/j.jmb.2012.04.002; PMID: 22484364
- Bowden TA, Baruah K, Coles CH, Harvey DJ, Yu X, Song BD, Stuart DI, Aricescu AR, Scanlan CN, Jones EY, et al. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J Am Chem Soc 2012; 134:17554 - 63; http://dx.doi.org/10.1021/ja306068g; PMID: 23025485
- Shibata-Koyama M, Iida S, Misaka H, Mori K, Yano K, Shitara K, Satoh M. Nonfucosylated rituximab potentiates human neutrophil phagocytosis through its high binding for FcgammaRIIIb and MHC class II expression on the phagocytotic neutrophils. Exp Hematol 2009; 37:309 - 21; http://dx.doi.org/10.1016/j.exphem.2008.11.006; PMID: 19218011
- Zhou Q, Shankara S, Roy A, Qiu H, Estes S, McVie-Wylie A, Culm-Merdek K, Park A, Pan C, Edmunds T. Development of a simple and rapid method for producing non-fucosylated oligomannose containing antibodies with increased effector function. Biotechnol Bioeng 2008; 99:652 - 65; http://dx.doi.org/10.1002/bit.21598; PMID: 17680659
- McGreal EP, Rosas M, Brown GD, Zamze S, Wong SY, Gordon S, Martinez-Pomares L, Taylor PR. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 2006; 16:422 - 30; http://dx.doi.org/10.1093/glycob/cwj077; PMID: 16423983
- Kogelberg H, Tolner B, Sharma SK, Lowdell MW, Qureshi U, Robson M, Hillyer T, Pedley RB, Vervecken W, Contreras R, et al. Clearance mechanism of a mannosylated antibody-enzyme fusion protein used in experimental cancer therapy. Glycobiology 2007; 17:36 - 45; http://dx.doi.org/10.1093/glycob/cwl053; PMID: 17000699
- Geijtenbeek TB, Kwon DS, Torensma R, van Vliet SJ, van Duijnhoven GC, Middel J, Cornelissen IL, Nottet HS, KewalRamani VN, Littman DR, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 2000; 100:587 - 97; http://dx.doi.org/10.1016/S0092-8674(00)80694-7; PMID: 10721995
- Van Patten SM, Hughes H, Huff MR, Piepenhagen PA, Waire J, Qiu H, Ganesa C, Reczek D, Ward PV, Kutzko JP, et al. Effect of mannose chain length on targeting of glucocerebrosidase for enzyme replacement therapy of Gaucher disease. Glycobiology 2007; 17:467 - 78; http://dx.doi.org/10.1093/glycob/cwm008; PMID: 17251309
- Franco A, Damdinsuren B, Ise T, Dement-Brown J, Li H, Nagata S, Tolnay M. Human Fc receptor-like 5 binds intact IgG via mechanisms distinct from those of Fc receptors. J Immunol 2013; 190:5739 - 46; http://dx.doi.org/10.4049/jimmunol.1202860; PMID: 23616577
- Yu X, Vasiljevic S, Mitchell DA, Crispin M, Scanlan CN. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J Mol Biol 2013; 425:1253 - 8; http://dx.doi.org/10.1016/j.jmb.2013.02.006; PMID: 23416198
- Käsermann F, Boerema DJ, Rüegsegger M, Hofmann A, Wymann S, Zuercher AW, Miescher S. Analysis and functional consequences of increased Fab-sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One 2012; 7:e37243; http://dx.doi.org/10.1371/journal.pone.0037243; PMID: 22675478
- Bayry J, Bansal K, Kazatchkine MD, Kaveri SV. DC-SIGN and alpha2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc Natl Acad Sci U S A 2009; 106:E24 - , author reply E25; http://dx.doi.org/10.1073/pnas.0900016106; PMID: 19237553
- Crispin M, Yu X, Bowden TA. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc Natl Acad Sci U S A 2013; 110:E3544 - 6; http://dx.doi.org/10.1073/pnas.1310657110; PMID: 23929778
- Sondermann P, Pincetic A, Maamary J, Lammens K, Ravetch JV. General mechanism for modulating immunoglobulin effector function. Proc Natl Acad Sci U S A 2013; 110:9868 - 72; http://dx.doi.org/10.1073/pnas.1307864110; PMID: 23697368
- Nimmerjahn F, Ravetch JV. Analyzing antibody-Fc-receptor interactions. Methods Mol Biol 2008; 415:151 - 62; PMID: 18370153
- Barouch DH, Yang ZY, Kong WP, Korioth-Schmitz B, Sumida SM, Truitt DM, Kishko MG, Arthur JC, Miura A, Mascola JR, et al. A human T-cell leukemia virus type 1 regulatory element enhances the immunogenicity of human immunodeficiency virus type 1 DNA vaccines in mice and nonhuman primates. J Virol 2005; 79:8828 - 34; http://dx.doi.org/10.1128/JVI.79.14.8828-8834.2005; PMID: 15994776
- Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856 - 61; http://dx.doi.org/10.1126/science.1187659; PMID: 20616233
- Orcutt KD, Ackerman ME, Cieslewicz M, Quiroz E, Slusarczyk AL, Frangioni JV, Wittrup KD. A modular IgG-scFv bispecific antibody topology. Protein Eng Des Sel 2010; 23:221 - 8; http://dx.doi.org/10.1093/protein/gzp077; PMID: 20019028
- Feng Y, Gong R, Dimitrov DS. Design, expression and characterization of a soluble single-chain functional human neonatal Fc receptor. Protein Expr Purif 2011; 79:66 - 71; http://dx.doi.org/10.1016/j.pep.2011.03.012; PMID: 21453773
- Brown EP, Licht AF, Dugast AS, Choi I, Bailey-Kellogg C, Alter G, Ackerman ME. High-throughput, multiplexed IgG subclassing of antigen-specific antibodies from clinical samples. J Immunol Methods 2012; 386:117 - 23; http://dx.doi.org/10.1016/j.jim.2012.09.007; PMID: 23023091
