Abstract
A role for SUMOylation in the biogenesis and/or function of Box C/D snoRNPs has been reported, mediated via SUMO2 conjugation to the core snoRNP protein, Nop58. A quantitative proteomics screen, based on SILAC (stable-isotope labeling by amino acids in cell culture) and mass spectrometry using extracts prepared from cultured mammalian cells expressing either 6His-SUMO1 or -SUMO2, revealed that the snoRNP-related proteins Nop58, Nhp2, DKC1 and NOLC1 are amongst the main SUMO-modified proteins in the nucleolus. SUMOylation of Nhp2 and endogenous Nop58 was confirmed using a combination of in vitro and cell-based assays and the modified lysines identified by site-directed mutagenesis. SUMOylation of Nop58 was found to be important for high-affinity Box C/D snoRNA binding and the localisation of newly transcribed snoRNAs to the nucleolus. Here, these findings are reviewed and a model for understanding Nop58 SUMOylation in the context of Box C/D snoRNP biogenesis is presented. Given the essential role of snoRNPs in the modification of pre-ribosomal RNA, this work suggests that SUMO, snoRNPs and ribosome assembly, and thus cellular translation, growth and proliferation, may be linked via novel mechanisms which warrant further investigation.
Introduction
Many major biological processes, including central steps in the gene expression pathway, are underpinned by macromolecular complexes that contain both protein and RNA (ribonucleoprotein complexes; RNPs). For many RNPs, the biogenesis pathways leading to the mature RNP are complex and highly regulated. Often, the assembly of these complexes occurs in a separate cellular compartment to where the final function is carried out, possibly highlighting the importance of separating immature RNPs from the interaction partners of the mature RNP.
The ribosome is the most abundant RNP in cells, and as such its biogenesis pathway has been well-studied.Citation1,Citation2 In eukaryotic cells, the bulk of ribosome assembly occurs in the nucleolus, which likely minimizes premature translation of nucleoplasmic and cytoplasmic mRNAs. The nucleolus is the major subnuclear compartment and its primary role is to produce the 18S and 28S pre-ribosomal RNAs (rRNAs) that are complexed with ribosomal proteins prior to the export of near-mature 40S and 60S ribosomal subunits to the cytoplasm (reviewed in ref. Citation3). Small nucleolar RNPs (snoRNPs) mediate the modification and processing of pre-rRNAs. snoRNP-mediated pre-rRNA modification is essential for ribosome production, translation and cell growth, with snoRNPs accounting for ∼10% of the mass of 90S pre-ribosomal particles and depletion of individual snoRNP components usually causing lethality in yeast.Citation4–Citation7
The biogenesis of snoRNPs occurs mainly in the nucleoplasm and involves a number of different, multi-protein complexes. In contrast, the mature snoRNP contains a single snoRNA and a specific set of four core proteins, which together maintain the structure, localization and stability of the snoRNA. Several hundred snoRNAs have been identified and classified according to conserved “Box C/D” or “Box H/ACA” motifs.Citation5 The core proteins of Box C/D snoRNPs are 15.5K/nhpx, Nop58, Nop56 and fibrillarin, whereas for Box H/ACA snoRNPs these are Nhp2, Gar1, Nop10 and DKC1.Citation4–Citation7 Most Box C/D snoRNPs mediate 2′-O-ribose methylation (via the methyltransferase, fibrillarin), and most Box H/ACA snoRNPs the pseudouridylation (via the pseudouridylase, DKC1) of pre-rRNA. The main exception is a subset of Box C/D snoRNPs (such as the U3 snoRNP) that carry out specific pre-rRNA cleavage events.Citation4–Citation7 Although the snoRNP biogenesis pathway has been well-studied, a role for post-translational modifications (PTMs) in this process had not yet been identified. Our recent work established a role for modification by SUMO (small ubiquitin-like modifier) in the biogenesis and/or function of human snoRNPs.Citation8 In particular, we discovered that Nop58 and Nhp2, core proteins of Box C/D and H/ACA snoRNPs respectively, are targets for SUMOylation in the nucleolus.
The Search for Nucleolar SUMO Targets using Stable-Isotope Labeling by Amino Acids in Cell Culture (SILAC)-Based Mass Spectrometry (MS)
In human cells, SUMO1 and SUMO2/3 (with SUMOs 2 and 3 being almost identical) are ∼10 kDa proteins that can be covalently attached to lysine residues in target proteins, via an enzymatic cascade analogous to ubiquitination and involving ATP, and E1, E2 and E3 enzymes.Citation9 SUMO2/3 can be linked in chains that can be terminated by SUMO1.Citation9 Hundreds of SUMO targets have been identified, and are implicated in a wide range of cellular processes, including nucleo-cytoplasmic transport, transcription and DNA repair. The mechanistic consequences of SUMOylation for many protein targets remain unknown, but in general SUMO conjugation affects molecular interactions that in turn alter the localization, activity and/or stability of the target.Citation9 The removal of SUMO from a target protein is mediated by sentrin-specific proteases (SENPs; 1–3 and 5–7 in humans).Citation10 The observation that SENP3 and SENP5 localise to the nucleolusCitation11–Citation13 suggests that a subset of nucleolar proteins may require deSUMOylation for proper functioning.
It was known that nucleoli contained significant levels of SUMO and consistent with this our quantitative immunofluorescence microscopy analysis revealed that ∼6% of nuclear SUMO resides in the nucleolus in HeLa cells.Citation8 Future work will determine if the level of nucleolar SUMO varies according to factors such as cell type, cell cycle stage, stress or disease. However, as proteomic analysis has shown that over 5,000 proteins can co-purify with human nucleoli,Citation14 it was hard to predict what the most likely nucleolar substrates for SUMOylation might be. We therefore conducted an unbiased, quantitative, MS-based proteomics screen for nucleolar SUMOylated proteins.Citation8 To do this, we used stable isotope labeled nucleolar extracts from HeLa cells stably expressing either 6His-tagged SUMO1 or -SUMO2, and enriched for SUMO-conjugated proteins by affinity purification using Ni2+-NTA resin ().Citation15,Citation16
The advantages of using SILAC-based technology combined with affinity purification include: (1) the metabolic labeling of proteins with different (light, medium or heavy; L, M or H) isotopes of arginine and lysine is relatively straightforward, (2) labeling cultured cells allows samples to be mixed prior to lysis, which minimizes experimental variation between samples, (3) the screen is unbiased, (4) quantitation is accurate because it is based on multiple ratios (H/L or M/L) of tryptic peptide pairs per protein and (5) the use of isotope ratios enables discrimination between proteins that bind either specifically, or non-specifically, to the Ni2+-NTA resin (refer to ref. Citation17 and Citation18 for discussion of SILAC-based pulldown experiments).
A particular challenge of SILAC-based quantitative proteomics is the interpretation of the unavoidably large, but information-rich, datasets. It is important to decide on a set of criteria for filtering the data to decide which prospective ‘hits’ merit downstream functional characterization. We focused on three main parameters derived from the MaxQuantCitation19 analysis of eluate samples to narrow down the list of potential SUMO substrates. Namely, the log2 (ratio M/L or H/L) value (which is >0 for SUMO substrates), “significance” of the ratio value (where smaller values indicate more likely SUMO substrates) and sequence coverage (higher percentages correspond to a higher number of quantified peptides per protein). Using these parameters and excluding SUMO1 and 2, we identified 18 and 10 potential SUMO1 and SUMO2 substrates, respectively, and observed that the three best candidates were all snoRNP proteins, specifically Nop58, Nhp2 and DKC1. The snoRNP transport/assembly factor NOLC1 also emerged as a potential substrate, albeit with lower sequence coverage.
Although subsequent characterization focused on Nhp2 and Nop58 SUMOylation, a number of other potentially interesting proteins were identified, such as the 3′ to 5′ exosome component EXOSC10 (or Pm/Scl-100), which is involved in mRNA degradation, surveillance and export. EXOSC10 also plays a role in 5.8S pre-rRNA maturation and replication-dependent histone mRNA degradation, and predominantly localises to the nucleolus and nucleoplasm.Citation20–Citation26 Furthermore, autoantibodies against EXOSC10 are intimately related to the severity of disease in patients with scleroderma.Citation27,Citation28 A relationship between the exosome, the nucleolus and SUMO has not previously been discovered.
Another dimension of our analysis involved comparing the proteomes of cells stably expressing either 6HisSUMO1 or 2 with the parental HeLa cells, i.e., the pulldown “inputs” (). This fourth parameter enabled identification of proteins that are either upregulated or stabilized, by the overexpression of exogenous His-tagged SUMO. These proteins could be either directly modified by SUMO or SUMO may instead be involved in an upstream pathway that leads to an increase in protein levels. For example, FHL1 was identified in our screen as a putative SUMO target but also appears to be upregulated in 6HisSUMO1-expressing cells. FHL1 mediates protein-protein interactions important for skeletal muscle development and function, and FHL1 mutations are linked with myopathies.Citation29 Direct SUMOylation of a FHL1 isoform has been shown, and is important for regulating its transcriptional activity.Citation30 We suggest that SUMOylation may also be involved in regulating FHL1 levels and that FHL1 may play a role in the nucleolus. We also observed a dramatic increase in the level of the key tumor suppressor protein CDKN2A (also called p14ARF) in cells stably expressing 6HisSUMO2. CDKN2A is known to localise to the nucleolus and possesses both p53-dependent and -independent roles in cell proliferation and growth control.Citation31,Citation32 Since CDKN2A is not a likely SUMO target, based on both experimental evidence and sequence analysis, it is likely that we may here have uncovered a novel pathway that involves SUMOylation of upstream targets and leads to increased CDKN2A levels.
Focussing on Two of the Novel Nucleolar SUMO Substrates: Nop58 and Nhp2
Following identification of snoRNP proteins as putative SUMO substrates, we went on to confirm these modifications and identify the targeted lysines using a variety of different experimental approaches. These included in vitro SUMOylation assays, cell-based transfection assays, primary sequence analysis and site-directed mutagenesis. We identified lysine residues K467 and K497 in Nop58, and K5 in Nhp2, as the main sites of SUMOylation. Furthermore, we were able to detect SUMOylated endogenous Nop58 in non-stressed cells in the absence of SUMO overexpression. This result is important to establish the physiological relevance of the modification but is often difficult to achieve due to non-specific SENP activity following cell lysis and the small ratio of modified to unmodified substrate. These features also often preclude the identification of functional consequences of SUMOylation for a given protein target, especially when combined with the challenge of selecting the “correct” experiment out of the myriad possible experiments that will reveal the functional consequence of SUMO conjugation. Indeed, initial experiments to identify the role of Nop58 SUMOylation revealed that it was not important for the proper subcellular localization of Nop58 to Cajal bodies (CBs) and nucleoli, and only modestly influenced association with one of the other core Box C/D snoRNP proteins, fibrillarin. However, we discovered that Nop58 SUMOylation was instead important for high-affinity binding to Box C/D snoRNAs and for the accumulation of newly transcribed Box C/D snoRNAs in nucleoli.
These novel roles for SUMO conjugation were discovered firstly by immunoprecipitation of wild-type (WT) and non-SUMOylatable (2mut) Nop58-GFP, followed by quantitative PCR to show that the levels of co-purifying snoRNAs (namely U3, U8, U13 and U14) were reduced for 2mutNop58-GFP. Secondly, we examined the localization of newly transcribed U3 snoRNAs using fluorescent in situ hybridization (FISH) in cells in which most endogenous Nop58 was replaced by either WT- or 2mutNop58-GFP. This revealed that newly transcribed snoRNA is not efficiently targeted to the nucleolus when the majority of Nop58 present is not SUMOylated. Instead, these snoRNAs remained in nucleoplasmic foci that likely correspond to transcription sites.Citation33 In these cells, 2mutNop58-GFP still exhibited nucleolar localization. We suggest that the maturation and association of snoRNAs with core Box C/D snoRNP proteins is prevented in the absence of Nop58 SUMOylation due to the inefficient recycling of assembly factors. It remains to be determined if an absence of Nhp2 SUMOylation will exhibit similar defects in Box H/ACA snoRNP biogenesis, or if SUMOylation of snoRNP components is important for their downstream function in pre-rRNA processing.
Mapping Nop58 SUMOylation Onto the Box C/D snoRNP Biogenesis Pathway
snoRNP biogenesis is highly intricate, involving macromolecular complexes that mediate specific protein/RNA folding and interaction events at a variety of subcellular locations.Citation4–Citation7 It will be important to pinpoint when Nop58 SUMOylation occurs during Box C/D snoRNP biogenesis, and we have developed a model (shown in ) that summarizes our current understanding of when this might occur, based on the following observations: (A) The majority of SUMOylated Nop58 accumulates in the nucleolus,Citation8 (B) The amount of endogenous SUMOylated Nop58 increases after siRNA-mediated knockdown of either one or both, nucleolar SENPs,Citation8 (C) Nop58 is assembled into snoRNPs in the nucleoplasm/CBs but can shuttle continually between the nucleoplasm and nucleolus,Citation4 (D) SUMOylation enzymes localise predominantly to the nucleoplasm (but note that a small fraction can be cytoplasmic and a role for SUMO in the cytoplasm has been established),Citation9,Citation34–Citation36 (E) Nucleolar Nop58 is more stable than nucleoplasmic Nop58 (Boisvert FM, Lamond AI, data not shown) and SENP3/5 knockdown increases total Nop58 levels,Citation8 and (F) Nop58 may also be a target for ubiquitination and proteasomal degradation, which likely occurs in the nucleoplasm (Belinda J. Westman and Angus I. Lamond, data not shown).Citation37
In the model (), newly translated Nop58 is rapidly imported into the nucleoplasm and accumulates in CBs. Nop58 associates with snoRNP assembly factors and core Box C/D snoRNP proteins. SUMOylation occurs either in the nucleoplasm, or in CBs or both, and promotes interactions with assembly factors required for restructuring events supporting stable association of snoRNP proteins with snoRNAs.Citation38 The mature complex subsequently accumulates in the nucleolus, where one or more of the SUMO molecules are deconjugated from Nop58 by SENP3 and/or 5. SUMOylation may act as a signalling “tag” for snoRNPs that have been assembled correctly, with defects preventing SUMOylation and allowing the opportunity for Nop58 ubiquitination and subsequent proteasomal degradation in the nucleoplasm. This model will inevitably be refined as more experiments are performed that confirm or disprove particular components. For example, the ability of Nop58 to be deSUMOylated by other SENPs besides the nucleolar SENP3 and SENP5, such as the nucleoplasmic SENP1, 6 or 7,Citation10 may need to be incorporated into this model.
Is the Highly Charged C-Terminal Nop58 Tail a “Hotspot” for Post-translational Modifications (PTMs)?
The SUMOylation sites (K467 and K497) are both located towards the C-terminus of Nop58, in a “tail” region rich in lysine and glutamate residues. The biological role of this tail is unclear, although it is conserved from yeast to human, and a similar tail region is present in other snoRNP proteins, including Nop56 and DKC1. Furthermore, a sequence similarity search for the Nop58 tail region (aa 436–529) with the BLAST (Basic Local Alignment Search Tool) programCitation39 reveals that similar K/E-rich regions can be identified in other proteins, including microtubule-associated protein 1B, chromatin-assembly factor 1, WD repeat containing protein 87, eukaryotic translation initiation factor 5B and ubiquitin carboxyl-terminal hydrolase 42. What role might this K/E-rich region play in these otherwise unrelated proteins?
We suggest that one feature of this charged region is to receive PTMs, which in turn regulate the stability and/or function of the corresponding protein. This idea is analogous to the N-terminal tail regions of histones, which can be targeted by different combinations of PTM machineries. The resultant amino acid modifications are able to modulate histone function by affecting protein-protein or protein-DNA interactions.Citation40
Alongside our report that Nop58 K467 and K497 residues are SUMOylated, Matic et al. published their findings that S502 in Nop58 is phosphorylated and is required for efficient SUMOylation of K497.Citation41 In addition, we have preliminary data suggesting that this region can also be ubiquitinated (Westman BJ, Lamond AI, data not shown). Indeed, several putative SUMOylation consensus site sequences can be identified within many of the other K/E-rich regions from the proteins mentioned above. Follow-up work will aim to investigate if these regions are modified by SUMO and/or other PTMs. However, we expect that only a subset of these K/E-rich regions have evolved as PTM-target sites, based on our finding that endogenous Nop56 is a poor substrate for SUMOylation compared to Nop58. Nop56 (another core Box C/D snoRNP protein) shares ∼40% sequence identity with Nop58 and also possesses a C-terminal, K/E-rich tail region. It will be interesting to determine if the lack of Nop56 SUMOylation is directly due to the primary sequence of the Nop56 tail, or rather due indirectly to features such as the position, or interactions, of the Nop56 tail within the structure of the snoRNP that might preclude accessibility by the SUMOylation machinery.
Understanding Nop58 SUMOylation at a Molecular Level
Our work raises many questions about the molecular detail of Nop58 SUMOylation and its effect on snoRNA binding. Firstly, what is the precise architecture of the SUMO moieties attached to Nop58, and how variable is this arrangement between different Nop58 molecules ()? It is likely that at least one lysine has multiple SUMOs attached, since at least three bands were detected by western blotting that correspond to SUMOylated Nop58 after SENP3/5 depletion and only two SUMOylated lysines (K467 and K497) exist. Further, we suggest that Nop58 is predominantly attached to SUMOs 2/3, since only SUMO2/3 can form chains, and because SENP3/5 display specific isopeptidase activity toward SUMO2/3.Citation10 Secondly, is the effect of SUMOylation on snoRNA binding direct or indirect? For example, SUMOylation may change the structural conformation of Nop58, or provide additional binding interfaces that result in an increase in snoRNA binding affinity. Alternatively, SUMOylation of Nop58 may promote interactions with particular assembly factors that are necessary for the snoRNP to adopt the optimal structural arrangements for high-affinity snoRNA binding. Interestingly, a recent report has shown that Hsp90 can stabilize SENP3 under mild oxidative stress by preventing its ubiquitination and subsequent degradation.Citation42 Given that one function of Hsp90 is as a snoRNP assembly factor,Citation43,Citation44 this report provides another potential link between snoRNP biogenesis and SUMOylation.
Nop58 and SUMO: What are the Upstream Regulators?
It will be important to understand the pathway of Nop58 SUMOylation within a cellular context, particularly if it is regulated by factors such as growth conditions, cell/tissue type or cell cycle stage. It is likely that a relationship exists between Nop58 SUMOylation, ribosome biogenesis and cellular translation, and this might be revealed by investigating pre-rRNA processing in cells expressing mainly non-SUMOylatable Nop58. The phosphorylated S502 in Nop58 is situated within a consensus site for casein kinase II (CK2),Citation41,Citation45 which is an ubiquitous serine/threonine kinase with hundreds of cellular substrates, including the nucleolar proteins NOLC1 and B23. Both increased expression and increased activity of CK2 have been linked to human cancers.Citation45 It will be interesting to examine if CK2 activity regulates Nop58 phosphorylation and therefore SUMOylation, which may in turn affect translation and cellular proliferation. The elucidation of the entire Nop58 SUMOylation pathway is still not complete and we anticipate other molecules involved in this pathway await discovery. For example, is either the dephosphorylation or SUMOylation, of Nop58 mediated by specific phosphatases or E3 SUMO ligases, respectively? Preliminary evidence suggests that the splicing factor, SF2/ASF, is a potential E3 SUMO ligase candidate since it was recently reported to promote Nop58 SUMOylation.Citation46
Outlook and Perspectives
The work we have summarized here represents one of the first systematic, unbiased screens for PTM-modified nucleolar targets in general, and SUMO targets in particular. Although so far follow-up work has concentrated on understanding the relationship between SUMO modification and snoRNP biogenesis/function, future research to evaluate other candidate SUMO target proteins, such as CDKN2A and EXOSC10, may also lead to novel findings. Nonetheless, the discovery that Nop58 SUMOylation is required for high-affinity snoRNA binding provides a new dimension to our current understanding of the mechanisms involved in Box C/D snoRNP biogenesis. We expect that further investigations will shed light on Nop58 SUMOylation at both the molecular and cellular levels, as well as whether or not SUMOylation is important for the assembly of Box H/ACA snoRNPs via targeting the core proteins, Nhp2 and DKC1. Finally, it will be revealing to better understand the interplay between SUMOylation, phosphorylation and ubiquitination of Nop58, and to discover if these regulatory PTMs are exploited more generally for other proteins that also possess similar K/E-rich regions to that which is targeted for SUMOylation in Nop58.
Figures and Tables
Figure 1 Summary of SILAC-based quantitative proteomics screen for nucleolar SUMO targets, illustrating the nature of the different eluate and input ratios that can be obtained following data analysis. Parental HeLa cells (black) and HeLa cells stably expressing either 6HisSUMO1 (S1; orange) or 6HisSUMO2 (S2; purple) were grown in media containing isotopically distinct R/K amino acids (light, medium or heavy; L, M or H). Equal numbers of cells were combined, input samples taken and nucleoli (white ovals) purified prior to denaturing pulldowns using Ni2+-NTA agarose. Mixing cell populations prior to fractionation and protein analysis avoids differential protein extraction from separate samples, which could affect quantitation and ensures peptide detection, measurement and comparison is made on samples from the same MS run. Bead eluates and inputs were subjected to SDS-PAGE, in-gel trypsin digestion and high-resolution mass spectrometry. Peptide ratios were quantified using MaxQuant (v1.0.13.13).Citation19 Input proteins have been classified as either “bead contaminants (circles)”, “proteins sensitive to SUMO overexpression (rounded rectangles)” or “SUMO targets (stars)”. The possible eluate and input ratios for each class are shown in the bottom two rows of the Figure. Note that proteins that are present at higher levels in the input samples because of the overexpression of 6HisSUMO1 or -2 could result in high log2 (eluate ratios), due either to direct SUMO modification, or to non-specific interactions with the Ni2+-NTA agarose.
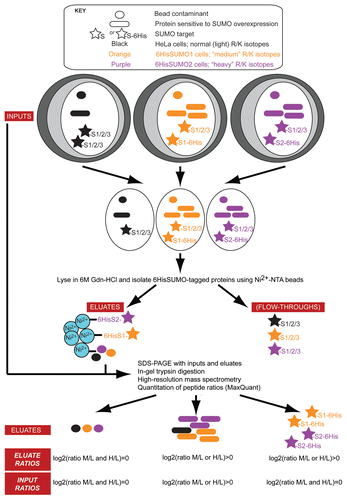
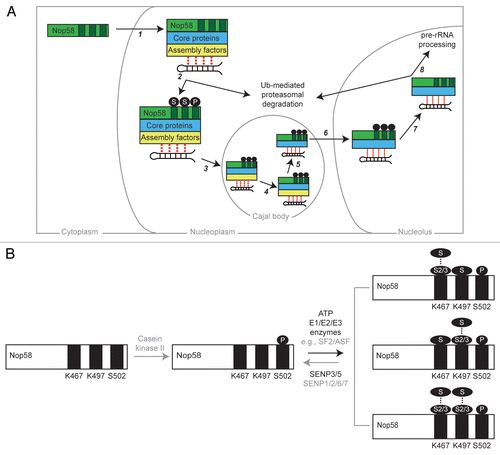
Figure 2 Schematic models summarising our current understanding of the role of Nop58 post-translational modifications (PTMs) in Box C/D snoRNP biogenesis (A), and the steps involved in these modifications on a molecular level (B). (A) The main steps (numbered 1–8) in Box C/D snoRNP biogenesis are summarized and their likely subcellular location (cytoplasm, nucleoplasm, Cajal body or nucleolus) indicated. Note that for some steps, the precise location requires further characterization. (1) Nop58 is imported into the nucleoplasm and associates with the four core snoRNP proteins (fibrillarin, 15.5K/nhpx and Nop56; blue) and a Box C/D snoRNA (shown as a stem-loop structure) via the action of a number of assembly/chaperone proteins (yellow). (2) During the assembly of the immature Box C/D snoRNP, Nop58 becomes phosphorylated (P) on S502 and SUMOylated (S) on K467 and K497 (modification sites shown as dark rectangles). SUMO may act as a tag to signal correct snoRNP assembly, with a lack of SUMOylation leading to ubiquitination (Ub) and subsequent proteasomal degradation in the nucleoplasm. (3) The immature snoRNP undergoes final assembly and processing steps in Cajal bodies, which in (4), lead to stabilization of the snoRNPCitation38,Citation47 (as indicated by a transition from dotted to solid red lines). (5) Assembly factors leave the mature Box C/D snoRNP. (6) The mature snoRNP accumulates in the nucleolus via the action of transport factors such as NOLC1. (7) Enzymes such as SENP3/5 deSUMOylate Nop58. It is possible that Nop58 is also dephosphorylated. (8) The mature, nucleolar Box C/D snoRNP participates in pre-rRNA processing and will likely undergo degradation in the nucleoplasm. (B) Depiction of the enzymes involved in PTM of Nop58, and the possible architecture of the resultant protein molecules. Grey text/arrows represent the reactions/enzymes that are more speculative than those shown in black. Nop58 undergoes casein kinase II-mediated phosphorylation of S502, which leads to SUMOylation of K467 and K497. SUMOylation may involve the action of E3 SUMO ligases such as SF2/ASF. The resultant Nop58 molecules contain SUMO chains (dots) either on K467 (top) or K497 (middle), or both K467 and K497 (bottom). Whilst the chains must contain predominately SUMO2/3, SUMO1 could be present at the end of the SUMO chain, or be attached as a single molecule to the other, non-SUMO-chain-conjugated lysine. The heterogeneity of the modified Nop58 populations is not known. Finally, deSUMOylation of Nop58 occurs via the action of SENP3/5, and possibly the other, non-nucleolar SENPs (1, 2, 6 or 7).
Acknowledgements
We thank Séverine Boulon and Mark Larance for critical reading of the manuscript. This work was supported by grants from the Wellcome Trust (083524/Z/07/Z) and MRC (69159) to A.I.L., and by funding from the UK RASOR network and by the EU networks EURASNET (LSHG-CT-2005-518238) and PROSPECTS (HEALTH-F4-2008-201648). A.I.L. is a Wellcome Trust Principal Research Fellow and B.J.W. is a Marie-Curie International Incoming Fellow (PIIF-GA-2008-219452).
References
- Henras AK, Soudet J, Gerus M, Lebaron S, Caizergues-Ferrer M, Mougin A, et al. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci 2008; 65:2334 - 2359; http://dx.doi.org/10.1007/s00018-008-8027-0
- Staley JP, Woolford JL Jr. Assembly of ribosomes and spliceosomes: Complex ribonucleoprotein machines. Curr Opin Cell Biol 2009; 21:109 - 118; PMID: 2698946; http://dx.doi.org/10.1016/j.ceb.2009.01.003
- Boisvert FM, van Koningsbruggen S, Navascues J, Lamond AI. The multifunctional nucleolus. Nat Rev Mol Cell Biol 2007; 8:574 - 585; http://dx.doi.org/10.1038/nrm2184
- Filipowicz W, Pogacic V. Biogenesis of small nucleolar ribonucleoproteins. Curr Opin Cell Biol 2002; 14:319 - 327 DOI: S0955067402003344
- Kiss T. Small nucleolar RNAs: An abundant group of noncoding RNAs with diverse cellular functions. Cell 2002; 109:145 - 148 DOI: S0092867402007183
- Reichow SL, Hamma T, Ferre-D'Amare AR, Varani G. The structure and function of small nucleolar ribonucleoproteins. Nucleic Acids Res 2007; 35:1452 - 1464; http://dx.doi.org/10.1093/nar/gkl1172
- Matera AG, Terns RM, Terns MP. Non-coding RNAs: Lessons from the small nuclear and small nucleolar RNAs. Nat Rev Mol Cell Biol 2007; 8:209 - 220; http://dx.doi.org/10.1038/nrm2124
- Westman BJ, Verheggen C, Hutten S, Lam YW, Bertrand E, Lamond AI. A proteomic screen for nucleolar SUMO targets shows SUMOylation modulates the function of Nop5/Nop58. Mol Cell 2010; 39:618 - 631; PMID: 2938476; http://dx.doi.org/10.1016/j.molcel.2010.07.025
- Geiss-Friedlander R, Melchior F. Concepts in sumoylation: a decade on. Nat Rev Mol Cell Biol 2007; 8:947 - 956; http://dx.doi.org/10.1038/nrm2293
- Yeh ET. SUMOylation and De-SUMOylation: Wrestling with life's processes. J Biol Chem 2009; 284:8223 - 8227; http://dx.doi.org/10.1074/jbc.R800050200
- Di Bacco A, Ouyang J, Lee HY, Catic A, Ploegh H, Gill G. The SUMO-specific protease SENP5 is required for cell division. Mol Cell Biol 2006; 26:4489 - 4498; http://dx.doi.org/10.1128/MCB.02301-05
- Gong L, Yeh ET. Characterization of a family of nucleolar SUMO-specific proteases with preference for SUMO-2 or SUMO-3. J Biol Chem 2006; 281:15869 - 15877; http://dx.doi.org/10.1074/jbc.M511658200
- Nishida T, Tanaka H, Yasuda H. A novel mammalian Smt3-specific isopeptidase 1 (SMT3IP1) localized in the nucleolus at interphase. Eur J Biochem 2000; 267:6423 - 6427 DOI: ejb1729
- Ahmad Y, Boisvert FM, Gregor P, Cobley A, Lamond AI. NOPdb: Nucleolar Proteome Database—2008 update. Nucleic Acids Res 2009; 37:181 - 184; http://dx.doi.org/10.1093/nar/gkn804
- Ong SE, Blagoev B, Kratchmarova I, Kristensen DB, Steen H, Pandey A, et al. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol Cell Proteomics 2002; 1:376 - 386
- Tatham MH, Rodriguez MS, Xirodimas DP, Hay RT. Detection of protein SUMOylation in vivo. Nat Protoc 2009; 4:1363 - 1371; http://dx.doi.org/10.1038/nprot.2009.128
- Boulon S, Ahmad Y, Trinkle-Mulcahy L, Verheggen C, Cobley A, Gregor P, et al. Establishment of a protein frequency library and its application in the reliable identification of specific protein interaction partners. Mol Cell Proteomics 2010; 9:861 - 879; PMID: 2871420; http://dx.doi.org/10.1074/mcp.M900517-MCP200
- Hubner NC, Bird AW, Cox J, Splettstoesser B, Bandilla P, Poser I, et al. Quantitative proteomics combined with BAC TransgeneOmics reveals in vivo protein interactions. J Cell Biol 2010; 189:739 - 754; PMID: 2872919; http://dx.doi.org/10.1083/jcb.200911091
- Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 2008; 26:1367 - 1372; http://dx.doi.org/10.1038/nbt.1511
- Schmid M, Jensen TH. The exosome: A multipurpose RNA-decay machine. Trends Biochem Sci 2008; 33:501 - 510; http://dx.doi.org/10.1016/j.tibs.2008.07.003
- Fomproix N, Hernandez-Verdun D. Effects of anti-PM-Scl 100 (Rrp6p exonuclease) antibodies on prenucleolar body dynamics at the end of mitosis. Exp Cell Res 1999; 251:452 - 464; http://dx.doi.org/10.1006/excr.1999.4578
- Schilders G, Raijmakers R, Raats JM, Pruijn GJ. MPP6 is an exosome-associated RNA-binding protein involved in 5.8S rRNA maturation. Nucleic Acids Res 2005; 33:6795 - 6804; PMID: 1310903; http://dx.doi.org/10.1093/nar/gki982
- Schilders G, van Dijk E, Pruijn GJM. C1D and hMtr4p associate with the human exosome subunit PM/Scl-100 and are involved in pre-rRNA processing. Nucleic Acids Res 2007; 35:2564 - 2572; http://dx.doi.org/10.1093/Nar/Gkm082
- Lejeune F, Li X, Maquat LE. Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating and exonucleolytic activities. Mol Cell 2003; 12:675 - 687 DOI: S1097276503003496
- Mullen TE, Marzluff WF. Degradation of histone mRNA requires oligouridylation followed by decapping and simultaneous degradation of the mRNA both 5′ to 3′ and 3′ to 5′. Genes Dev 2008; 22:50 - 65; PMID: 2151014; http://dx.doi.org/10.1101/gad.1622708
- Brouwer R, Pruijn GJ, van Venrooij WJ. The human exosome: An autoantigenic complex of exoribonucleases in myositis and scleroderma. Arthritis Res 2001; 3:102 - 106; PMID: 128886
- Reimer G. Autoantibodies against nuclear, nucleolar and mitochondrial antigens in systemic sclerosis (scleroderma). Rheum Dis Clin North Am 1990; 16:169 - 183
- Pollard KM, Reimer G, Tan EM. Autoantibodies in scleroderma. Clin Exp Rheumatol 1989; 7:57 - 62
- Shathasivam T, Kislinger T, Gramolini AO. Genes, proteins and complexes: The multifaceted nature of FHL family proteins in diverse tissues. J Cell Mol Med 2010; http://dx.doi.org/10.1111/j.1582-4934.2010.01181
- Wang J, Qin H, Liang J, Zhu Y, Liang L, Zheng M, et al. The transcriptional repression activity of KyoT2 on the Notch/RBP-J pathway is regulated by PIAS1-catalyzed SUMOylation. J Mol Biol 2007; 370:27 - 38; http://dx.doi.org/10.1016/j.jmb.2007.04.010
- Dominguez-Brauer C, Brauer PM, Chen YJ, Pimkina J, Raychaudhuri P. Tumor suppression by ARF: Gatekeeper and caretaker. Cell Cycle 2010; 9:86 - 89 DOI: 10350
- Lindstrom MS, Klangby U, Inoue R, Pisa P, Wiman KG, Asker CE. Immunolocalization of human p14(ARF) to the granular component of the interphase nucleolus. Exp Cell Res 2000; 256:400 - 410; http://dx.doi.org/10.1006/excr.2000.4854
- Verheggen C, Lafontaine DL, Samarsky D, Mouaikel J, Blanchard JM, Bordonne R, et al. Mammalian and yeast U3 snoRNPs are matured in specific and related nuclear compartments. EMBO J 2002; 21:2736 - 2745; http://dx.doi.org/10.1093/emboj/21.11.2736
- Rodriguez MS, Dargemont C, Hay RT. SUMO-1 conjugation in vivo requires both a consensus modification motif and nuclear targeting. J Biol Chem 2001; 276:12654 - 12659; http://dx.doi.org/10.1074/jbc.M009476200
- Azuma Y, Tan SH, Cavenagh MM, Ainsztein AM, Saitoh H, Dasso M. Expression and regulation of the mammalian SUMO-1 E1 enzyme. FASEB J 2001; 15:1825 - 1827
- Gill G. SUMO and ubiquitin in the nucleus: different functions, similar mechanisms?. Genes Dev 2004; 18:2046 - 2059; http://dx.doi.org/10.1101/gad.1214604
- Rockel TD, Stuhlmann D, von Mikecz A. Proteasomes degrade proteins in focal subdomains of the human cell nucleus. J Cell Sci 2005; 118:5231 - 5242; http://dx.doi.org/10.1242/jcs.02642
- Watkins NJ, Lemm I, Ingelfinger D, Schneider C, Hossbach M, Urlaub H, et al. Assembly and maturation of the U3 snoRNP in the nucleoplasm in a large dynamic multiprotein complex. Mol Cell 2004; 16:789 - 798; http://dx.doi.org/10.1016/j.molcel.2004.11.012
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol 1990; 215:403 - 410; http://dx.doi.org/10.1006/jmbi.1990.9999
- Munshi A, Shafi G, Aliya N, Jyothy A. Histone modifications dictate specific biological readouts. J Genet Genomics 2009; 36:75 - 88; http://dx.doi.org/10.1016/S1673-8527(08)60094-6
- Matic I, Schimmel J, Hendriks IA, van Santen MA, van de Rijke F, van Dam H, et al. Site-specific identification of SUMO-2 targets in cells reveals an inverted SUMOylation motif and a hydrophobic cluster SUMOylation motif. Mol Cell 2010; 39:641 - 652; http://dx.doi.org/10.1016/j.molcel.2010.07.026
- Yan S, Sun X, Xiang B, Cang H, Kang X, Chen Y, et al. Redox regulation of the stability of the SUMO protease SENP3 via interactions with CHIP and Hsp90. EMBO J 2010; http://dx.doi.org/10.1038/emboj.2010.245
- Boulon S, Marmier-Gourrier N, Pradet-Balade B, Wurth L, Verheggen C, Jady BE, et al. The Hsp90 chaperone controls the biogenesis of L7Ae RNPs through conserved machinery. J Cell Biol 2008; 180:579 - 595; http://dx.doi.org/10.1083/jcb.200708110
- Zhao R, Kakihara Y, Gribun A, Huen J, Yang G, Khanna M, et al. Molecular chaperone Hsp90 stabilizes Pih1/Nop17 to maintain R2TP complex activity that regulates snoRNA accumulation. J Cell Biol 2008; 180:563 - 578; http://dx.doi.org/10.1083/jcb.200709061
- Hanif IM, Shazib MA, Ahmad KA, Pervaiz S. Casein Kinase II: an attractive target for anti-cancer drug design. Int J Biochem Cell Biol 2010; 42:1602 - 1605; http://dx.doi.org/10.1016/j.biocel.2010.06.010
- Pelisch F, Gerez J, Druker J, Schor IE, Munoz MJ, Risso G, et al. The serine/arginine-rich protein SF2/ASF regulates protein sumoylation. Proc Natl Acad Sci USA 2010; 107:16119 - 16124; PMID: 2941313; http://dx.doi.org/10.1073/pnas.1004653107
- McKeegan KS, Debieux CM, Boulon S, Bertrand E, Watkins NJ. A dynamic scaffold of pre-snoRNP factors facilitates human box C/D snoRNP assembly. Mol Cell Biol 2007; 6782 - 6793; PMID: 17636026