Abstract
The nucleolus, the most prominent structure observed in the nucleus, is often called a “ribosome factory.” Cells spend an enormous fraction of their resources to achieve the mass-production of ribosomes required by rapid growth. On the other hand, ribosome biogenesis is also tightly controlled, and must be coordinated with other cellular processes. Ribosomal proteins and ribosome biogenesis factors are attractive candidates for this link. Recent results suggest that some of them have functions beyond ribosome biogenesis. Here we review recent progress on ribosome biogenesis factors, Ebp2 and Rrs1, in yeast Saccharomyces cerevisiae. In this organism, Ebp2 and Rrs1 are found in the nucleolus and at the nuclear periphery. At the nuclear envelope, these proteins interact with a membrane-spanning SUN domain protein, Mps3, and play roles in telomere clustering and silencing along with the silent information regulator Sir4. We propose that a protein complex consisting Ebp2, Rrs1 and Mps3 is involved in a wide range of activities at the nuclear envelope.
Ribosome Biogenesis and Surveillance
All life on earth depends on the ribosome for protein synthesis. The ribosome is a complicated ribonucleoprotein nanomachine and its biogenesis in eukaryotic cells is extraordinarily complex, requiring four different ribosomal RNAs (rRNAs; 25S/28S, 18S, 5.8S and 5S rRNA) and about 80 ribosomal proteins as the constituent materials. Another 200 non-ribosomal proteins and RNA molecules serve as processers, modifiers and assemblers (reviewed in refs. Citation1 and Citation2). The multistep maturation process occurs sequentially in the nucleolus, the nucleoplasm and the cytoplasm, where pre-ribosome particles are converted to functional 40S and 60S ribosomal subunits (reviewed in refs. Citation3 and Citation4). In rapidly proliferating yeast cells, rRNA accounts for approximately 80% of total cellular RNA, and 50% of RNA polymerase II transcription is devoted to ribosomal protein genes (reviewed in ref. Citation5). Thus, the process of ribosome biogenesis is both high-throughput and high-precision, requiring quality control and feedback mechanisms to adjust itself to demand.
It has been suggested that many ribosomal proteins have extra-ribosomal functions, which help them serve as sentinels for ribosome biogenesis (reviewed in ref. Citation6). One of the best known examples is the function that monitors ribosome imbalance in mammals. Ribosomal proteins like L11, L5, L23 and S7, interact with M/HDM2 (the mouse/human ortholog of E3 ligase responsible for the ubiquitination of p53). Under conditions that block transcription of rRNA or stall ribosome assembly, increased binding of these ribosomal proteins to M/HDM2 leads to p53 accumulation and cell-cycle arrest. L11 can also bind and inhibit c-Myc, which functions as an activator of all three RNA polymerases. In addition, several factors that are involved in ribosome maturation connect defects in ribosome maturation with p53 accumulation and subsequent cell-cycle arrest (reviewed in ref. Citation6). Finally, it was shown that bona fide ribosome biogenesis factors can have functions in various other cellular processes in yeast, such as DNA replication and cell polarityCitation7 (reviewed in ref. Citation8). Nonetheless, how ribosome synthesis itself is coordinated with other cellular mechanisms remains unclear.
Ebp2 and Rrs1 are Bona Fide Ribosome Biogenesis Factors
We have previously shown that Ebp2, yeast homolog of human Epstein-Barr virus nuclear antigen 1-binding protein 2, binds to Rrs1, and both are required for the maturation of 25S rRNA and the production of the 60S ribosomal subunit in yeast.Citation9,Citation10 These processes mainly occur in the nucleolus. It was demonstrated that Rrs1 and Rpf2 form a subcomplex with L5, L11 and 5S rRNA for the coordinated recruitment of these components to pre-60S ribosomal subunits.Citation11,Citation12 While Ebp2 and Rrs1 bind to each other, they may not always work together in ribosome biogenesis. The interaction between Rrs1 and Ebp2 is weaker than that between Rrs1 and Rpf2Citation13 and Ebp2 also has other binding partners with roles in ribosome biogenesis (our unpublished data). Consistently, an ebp2 mutation causes the accumulation of different species of rRNA precursors from those that accumulate upon Rrs1 depletion.Citation13,Citation14 Moreover, double staining of Ebp2 and Rrs1 showed that Rrs1 generally had a more peripheral distribution than Ebp2 in the nucleolus, reflecting their slightly different stages of function in ribosome biogenesis.Citation15
Perinuclear Localizations of Ebp2 and Rrs1 Mediated by a SUN Domain Protein Mps3
In several temperature-sensitive ebp2 and rrs1 mutants, each of which had defects in 25S rRNA maturation and in 60S ribosomal subunit production,Citation15-Citation17 the nuclear envelope became distorted into a nonspherical shape at the restrictive temperature. This was observed more rapidly than the defect in biogenesis of the 60S ribosomal subunit after the temperature shift-up. Fluorescence microscopy has shown that Ebp2 and Rrs1 are localized along the inner nuclear envelope at non-pore sites, as well as in the crescent-shaped nucleolus throughout the cell cycle (and, left). Moreover, the perinuclear signals are lost in the ebp2 and rrs1 mutants when the cells are placed at the restrictive temperature ().
Figure 1. Roles of Ebp2 and Rrs1 at the nuclear periphery and summary of phenotypes in each condition. (A) Ebp2 and Rrs1 localize to the perinuclear region through Mps3, and thereby serve a structural function in the nucleus. Ebp2, Rrs1 and Mps3 are associated with the silent information factor Sir4, and function in telomere clustering in S phase cells and silencing. (B) Mutant forms of Ebp2 and Rrs1 lose association with the nuclear periphery at the restrictive temperature, thereby leading to the loss of the integrity of the nuclear envelope. Telomeres remained localized to the nuclear envelope but lost the cluster formation in S phase cells and the silent domain organization. (C) The ectopic expression of Mps3-N′ leads Ebp2 and Rrs1 away from the nuclear periphery, thereby compromising their telomeric roles and the structural role at the nuclear periphery. Mps3-N′ also titrate Est1, a component of telomerase which is required for Ku-dependent telomere tethering in S phase, from the perinuclear binding sites.Citation21 (D) The lack of Sir4 does not affect the interaction of Ebp2 and Rrs1 with Mps3 and the perinuclear localization of Ebp2. Telomeres are detached from the nuclear periphery due to the absence of Sir4. The perinuclear Ebp2 and Rrs1 is sufficient for providing the structural role independently of telomeres. ONM, outer nuclear membrane; INM, inner nuclear membrane; M-N’, Mps3-N′; E/R, Ebp2 and Rrs1; NE, nuclear envelope; Tel., telomere; gray ellipse, unknown proteins constituting the perinuclear protein network; white circle, telomeric complex of Yku70/80 and telomerase.
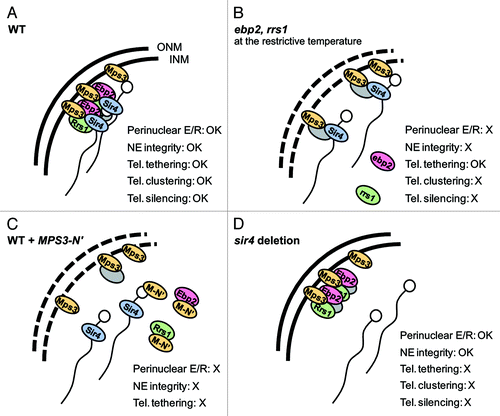
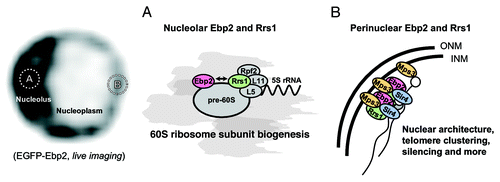
Figure 2. Two states of Ebp2 and Rrs1 in the nucleus. (A) Ebp2 and Rrs1 interact with each other. Freely diffusible forms of Ebp2 and Rrs1 in the nucleolus have essential functions in biogenesis of the 60S ribosomal subunit. Rrs1 and Rpf2 are necessary for incorporation of 5S rRNA and ribosomal proteins L5 and L11 into pre-ribosomes. (B) Ebp2 and Rrs1 form the complex with Mps3 at the nuclear periphery and act as a complex playing roles in the structural maintenance of the nucleus and the telomere clustering and silencing. Gray blank ellipse, unknown proteins constituting perinuclear protein network.
An earlier yeast two-hybrid screen for ligands of Schizosaccharomyces pombe Sad1, a founding member of the SUN (Sad1-UNC84) family, identified the S. pombe Ebp2 and Rrs1, as well as 23 other proteins located at the spindle pole body, the nuclear pore complex and the nuclear membrane.Citation18 SUN proteins are conserved across eukaryotes and are components of the inner nuclear membrane with the N-terminal domain in the nucleoplasm (reviewed in ref. Citation19). We hypothesized that the Sad1 ortholog in S. cerevisiae, Mps3,Citation20 might be responsible for Ebp2/Rrs1 positioning at the nuclear periphery. Using a yeast two-hybrid and in vitro pull-down assays, we demonstrated the interaction of nucleoplasmic N-terminal domain of Mps3 with Ebp2/Rrs1. In addition, coimmunoprecipitation of Mps3 demonstrated that Ebp2 and Rrs1 form a complex with Mps3 in vivo. We showed that the ectopic expression of the nucleoplasmic N-terminal domain of Mps3 (aa 1-153; Mps3-N′), which diffuses freely throughout the nucleoplasm,Citation21 antagonized the perinuclear localization of both Ebp2 and Rrs1, suggesting that targeting of Ebp2 and Rrs1 to the nuclear periphery is dependent on Mps3 (). Intriguingly, Mps3-N’ also provoked a non-spherical nuclear shape like that seen in the ebp2 and rrs1 mutants at restrictive temperature.
In higher eukaryotes, SUN proteins bind directly to the non-membrane protein lamin which self-associates via coiled-coil domains, providing the nucleus with a basic mechanical unit and supporting cross-talk from the cytoskeleton to the nuclear interior, possibly through the attachment or movement of chromatin domains (reviewed in ref. Citation19). The structural maintenance of the nuclear envelope has engendered new excitement because of its relevance to tissue integrity and premature aging diseases. Mutations in lamin A/C lead to altered mechanical properties of the nuclear laminaCitation22 and are linked to multiple types of dystrophies, collectively termed laminopathies (reviewed in refs. Citation19 and Citation23). Not only with the nuclear lamina, but SUN proteins play a critical role in nuclear envelope integrity by localizing outer nuclear membrane proteins, Nesprin-1 and -2, which link the nucleus to the cytoskeleton. It has been shown that Nesprin-1 and -2 are involved in the pathogenesis of Emery-Dreifuss muscular dystrophy patients (reviewed in refs. Citation19 and Citation23). Yeast has no lamin, but yeast Ebp2 possesses three coiled-coil domains and can form homodimers.Citation9 Thus the possibility exists that Ebp2 and Rrs1 are members of the perinuclear network of coiled-coil proteins including Esc1, Sir4 and Smc5/6 that contributes to the structural integrity of the nuclear envelope in yeast.
Ebp2 and Rrs1 Interact with Sir4 and are Required for the Telomere Clustering and Silencing
Yeast telomeres are anchored in clusters at the nuclear periphery. Anchoring is achieved through two partially redundant pathways, involving Sir4 and the Yku70/Yku80 heterodimer.Citation24,Citation25 The former pathway relies on the interaction of Sir4 with Mps3 and Esc1, a peripheral membrane anchor, while the anchoring of Ku depends on the interaction of a telomerase with Mps3 in S-phase cells, and an as yet unidentified anchor in G1 phase.Citation21,Citation26 The interaction of telomere-associated factors with nuclear envelope proteins is necessary to establish a specialized subnuclear compartment, in which telomeres and Sir factors are sequestered. Telomere position in vegetative yeast nuclei is maintained in a cell cycle-dependent manner.Citation21,Citation24-Citation27
We showed that both Ebp2 and Rrs1 interact with the C-terminal domain of Sir4 (), and conditional inactivation of either ebp2 or rrs1 interferes with both the clustering and silencing of yeast telomeres, while telomere tethering to the nuclear periphery remains intact (). Importantly, expression of an Ebp2-Mps3 fusion protein which is spatially limited to the nuclear periphery suppresses the defect of the ebp2 mutant for telomere clustering, but not its defects in growth or ribosome biogenesis. Thus, we conclude that the defects in telomere clustering can be dissociated from the defects in ribosome biogenesis for Ebp2 and Rrs1, even though both proteins are clearly implicated in both events. In addition to our findings, several recent papers addressed the clustering mechanism and the relation with tethering and silencing of telomeres. A screen using GFP-Rap1 to mark telomeric repeat sequences has identified various mutants defective in telomere clustering and/or the localization to the nuclear periphery.Citation28 Several mutants led to a similar telomeric clustering phenotype as ebp2 and rrs1, i.e., loss of clustering but not tethering. Intriguingly, a recent paper showed that overexpressing Sir3 leads to the hyperclustering of telomeres and silencing factors in foci localized away from the nuclear periphery, and could separate Sir3's role in telomere clustering from its role in silencing.Citation29 These results reinforce our own which identify distinct pathways for the clustering and anchoring of telomeres in yeast.
Ebp2 and Rrs1 as New Players of the Solar System
Warner and McIntosh have proposed three criteria for the ribosomal proteins which are truly acting in an extraribosomal capacity.Citation6 If we expand the application range to ribosome biogenesis factors, Ebp2 and Rrs1 meet these criteria, as follows: (1) both Ebp2 and Rrs1 bind specifically to a component of the cell, Mps3, which is not involved in ribosome biogenesis; (2) the interaction has a physiological effect on a cell; and (3) the function occurs independently of the ribosome biogenesis. The perinuclear Ebp2 and Rrs1 have specific functions which are separable from ribosome biogenesis roles of nucleolar Ebp2 and Rrs1. This finding raises the next question of whether the peripheral Ebp2 and Rrs1 function exclusively in telomere clustering and silencing. Instead, we propose that Ebp2 and Rrs1 act as a scaffolding or coordinator for various chromatin reactions, much like their ligand, Mps3. Indeed, Ebp2 and Rrs1 bind to the nucleoplasmic region of Mps3 independently of Sir4, and in addition, that Ebp2 is localized at the nuclear periphery in sir4Δ cells (). These results support the idea that perinuclear Ebp2 and Rrs1 can act with Mps3 independently of the presence of telomeres at the nuclear periphery.
The nuclear envelope is one of the key structures that regulates the positioning of various chromosomal loci in yeast. Recent studies showed that the damaged DNA is recruited to the nuclear envelope and that the relocation is important for the DNA double strand break (DSB) processing.Citation30 Both nuclear pores and the SUN domain protein Mps3 are thought to provide the landing sites to the persistent DSBs, whereas the latter also provides the site to telomeres.Citation21,Citation31-Citation33 It is expected that Mps3 works as a control hub for distinguishing and differently managing telomeres and DSBs, both of which contain chromosome ends and share many regulatory factors, such as the Ku complex, MRX (MRN) and Tel1 (ATM). Although many factors have been identified as targeting or regulating factors for repair or maintenance of telomeric ends, we know only a few proteins implicated on the side of the nuclear envelope. Intriguingly, ebp2 and rrs1 mutants show hypersensitivity to DNA damaging agents, such as hydroxyurea and methyl methanesulfonate, in some cases more severely than mps3Δ65-145 (Horigome et al., unpublished data). The result raises the possibility that the perinuclear complement of Ebp2 and Rrs1 contribute the function of Mps3 not only in telomere maintenance but in DNA repair.
Ebp2 is Modified with the Small Ubiquitin-Related Modifier (SUMO)
Although the telomere organization in the nucleus is tightly regulated in cell-cycle dependent manner, the nuclear peripheral localization of Ebp2 and Rrs1 persists throughout the cell-cycle, suggesting that perinuclear Ebp2 and Rrs1 may be controlled by post-translational modification. Indeed, Ebp2 is sumoylated and interacts with the PIAS-like SUMO E3 ligase Siz2 as well as with two SUMO-related proteins, Uls1/Ris1 and Wss1.Citation34 Although it was demonstrated that global perturbation of SUMO conjugation and deconjugation, impairs both the maturation and export of ribosomal subunits from the nucleus,Citation35 the sumoylation of Ebp2 may have other functions. First of all, sumoylation-defective ebp2 mutants show the same growth rate as wild-type cells, and secondly, the sumoylation-defective forms of Ebp2 retained positive interactions with ribosome assembly factors such as Nop12 and Loc1. In contrast to this, sumoylation-defective forms of Ebp2 completely lost their binding to Siz2, Uls1/Ris1 and Wss1. Recent studies have demonstrated the importance of these SUMO-recognizing partners of Ebp2 in telomere maintenance and DNA damage repair at the nuclear periphery. Ferreira et al. reported that Siz2 sumoylates both Yku70/80 and Sir4 in vivo and is required for telomere anchoring—at least in part due to the sumoylation of the yeast Ku complex.Citation26 Wss1 plays a role in removing SUMO and ubiquitin from proteins undergoing proteasomal degradation and genetically interacts with the Slx5-Slx8 SUMO-targeted ubiquitin ligase complex,Citation36,Citation37 which is implicated in the DSB relocation to the periphery and the repair.Citation30 Thus, the loss of interaction between Ebp2 and Siz2, Uls1 and Wss1, upon loss of sumoylation implicates Ebp2 in the pathway that positions telomeres in S phase and targets DSB to either nuclear pores or Mps3.
Ebp2, Rrs1 and SUN Proteins in Other Eukaryotes
Ebp2 and Rrs1 are highly conserved proteins and ribosome biogenesis is a conserved process within eukaryotic organisms. Human EBP2 and RRS1 are localized in the nucleolus, suggesting that their roles in ribosome biogenesis are conserved.Citation38,Citation39 It has been reported the nucleolar localization of human EBP2 is dependent on nucleostemin, which is a nucleolar protein preferentially expressed in actively proliferating cells, such as cancer cells and stem cells, and is involved in the ribosome biogenesis.Citation40 Thus, human EBP2 might connect ribosome biogenesis with cell proliferation.
While perinuclear localizations of EBP2 and RRS1 have not been reported to date except for budding yeast, we predict that the non-ribosomal functions of these proteins (which take place at the nuclear envelope in yeasts) are conserved in mammals and other species. As mentioned above, the yeast two-hybrid screen for S. pombe SUN protein Sad1 identified only two nucleolar proteins, the S. pombe Ebp2 and Rrs1.Citation18 Therefore, it seems that the interaction of perinuclear Ebp2 and Rrs1 with the unique SUN domain protein is conserved in fission yeast, although the role of this interaction has not been studied in S pombe. Human EBP2 and RRS1 are found associated with condensed chromosomes in mitosis.Citation39,Citation41,Citation42 Intriguingly, SUN proteins also bind to the peripheral edges of condensed human chromosomes,Citation43,Citation44 suggesting that human EBP2, RRS1 and SUN proteins also colocalize at least transiently.
A study on human EBP2 offers valuable insight for the functional modality of the protein in ribosome biogenesis.Citation45 Hirano et al. prepared a ribonucleoprotein-containing nuclear matrix fraction of HeLa cells which is biochemically defined as an insoluble structure as to detergent- and high salt-extraction followed by removal of chromatin (reviewed in ref. Citation46), and identified 83 proteins in the fraction by the peptide mass fingerprint (PMF). Coupled with many structural and RNA binding proteins (60 of 83 proteins), EBP2 and BXDC1 which is the human homolog of yeast Rpf2 were identified. Fluorescence microscopy and fluorescence recovery after photobleaching (FRAP) analyses showed that EBP2 and BXDC1 are more tightly associated with the nucleolus in an RNA-dependent manner, at least than nucleolar proteins B23, nucleolin and fibrillarin. Thus, it was proposed that EBP2 and BXDC1 are “dynamic scaffold” proteins which serve as a core structure for ribosome biogenesis.
While nucleoli appear to be detached from the nuclear envelope in higher eukaryotes, several studies revealed the existence of the structural links between the nuclear envelope and nucleoli. Transmission electron microscopy (TEM) analysis of the ribonucleoprotein-containing nuclear matrix fraction of HeLa cells, which includes EBP2 and BXDC1, revealed the existence of RNase-sensitive fibers that extend throughout the nucleus, forming continuous association between nucleoli and the nuclear lamina.Citation47 Given that the shape of the extracted nucleus was sensitive to RNase, it was long proposed that RNA would be an important structural component of the nucleus. Alternative links may also exist; in the germinal vesicle of Xenopus oocytes bundles of actin extend from nucleoli to the nuclear envelope,Citation48,Citation49 and lamin B1 appears to form an external scaffold for nucleoli.Citation50 In the case of budding yeast, the nucleolus forms a crescent-shaped structure that makes extensive contact with the nuclear envelope. It has been shown that a protein network including the inner nuclear membrane protein Src1 (also called Heh1) and Sir2 stabilizes the highly repetitive ribosomal DNA sequences.Citation51 Src1 also functions in subtelomeric gene expression and is embedded functionally in a network of factors, which participate in transcription export (TREX) complex-dependent mRNA export through the nuclear pore complexes.Citation52 We speculate that yeast perinuclear- and nucleolar-Ebp2/Rrs1 are conducive to a dynamic network between RNAs and proteins such as Mps3 contributing to subnuclear structure.
Concluding Remarks
Ebp2 and Rrs1 are conserved nuclear proteins that act at the nuclear membrane with the SUN protein to maintain a spherical nuclear structure and probably to control chromatin repair and protection. Yet, we are only at the beginning of uncovering its function in processes such as telomere maintenance. Since the SUN proteins impact a wide range of activities at the nuclear envelope directly or indirectly, the understanding of the relationship between SUN proteins and Ebp2/Rrs1 is likely to shed light on the true nature of genome organization. Further studies will examine whether the nucelolar- and the perinuclear-Ebp2 and Rrs1 functions are related.
| Abbreviations: | ||
| rRNA | = | ribosomal RNA |
| SUN | = | Sad1-UNC84 homology |
| SUMO | = | small ubiquitin-related modifier |
| DSB | = | DNA double strand break |
Acknowledgments
We regret the omission of many important references due to space constraint. We thank Susan M. Gasser for support and critical reading. This work was supported by grants from the Japanese Ministry MEXT and JSPS. C.H. was financially supported by JSPS Research Fellowship for Young Scientists and Marie Curie International Incoming Fellowship.
References
- Henras AK, Soudet J, Gérus M, Lebaron S, Caizergues-Ferrer M, Mougin A, et al. The post-transcriptional steps of eukaryotic ribosome biogenesis. Cell Mol Life Sci 2008; 65:2334 - 59; http://dx.doi.org/10.1007/s00018-008-8027-0; PMID: 18408888
- Staley JP, Woolford JL Jr. Assembly of ribosomes and spliceosomes: complex ribonucleoprotein machines. Curr Opin Cell Biol 2009; 21:109 - 18; http://dx.doi.org/10.1016/j.ceb.2009.01.003; PMID: 19167202
- Tschochner H, Hurt E. Pre-ribosomes on the road from the nucleolus to the cytoplasm. Trends Cell Biol 2003; 13:255 - 63; http://dx.doi.org/10.1016/S0962-8924(03)00054-0; PMID: 12742169
- Panse VG, Johnson AW. Maturation of eukaryotic ribosomes: acquisition of functionality. Trends Biochem Sci 2010; 35:260 - 6; http://dx.doi.org/10.1016/j.tibs.2010.01.001; PMID: 20137954
- Warner JR. The economics of ribosome biosynthesis in yeast. Trends Biochem Sci 1999; 24:437 - 40; http://dx.doi.org/10.1016/S0968-0004(99)01460-7; PMID: 10542411
- Warner JR, McIntosh KB. How common are extraribosomal functions of ribosomal proteins?. Mol Cell 2009; 34:3 - 11; http://dx.doi.org/10.1016/j.molcel.2009.03.006; PMID: 19362532
- Yamada H, Horigome C, Okada T, Shirai C, Mizuta K. Yeast Rrp14p is a nucleolar protein involved in both ribosome biogenesis and cell polarity. RNA 2007; 13:1977 - 87; http://dx.doi.org/10.1261/rna.553807; PMID: 17804645
- Dez C, Tollervey D. Ribosome synthesis meets the cell cycle. Curr Opin Microbiol 2004; 7:631 - 7; http://dx.doi.org/10.1016/j.mib.2004.10.007; PMID: 15556036
- Tsujii R, Miyoshi K, Tsuno A, Matsui Y, Toh-e A, Miyakawa T, et al. Ebp2p, yeast homologue of a human protein that interacts with Epstein-Barr virus Nuclear Antigen 1, is required for pre-rRNA processing and ribosomal subunit assembly. Genes Cells 2000; 5:543 - 53; http://dx.doi.org/10.1046/j.1365-2443.2000.00346.x; PMID: 10947841
- Tsuno A, Miyoshi K, Tsujii R, Miyakawa T, Mizuta K. RRS1, a conserved essential gene, encodes a novel regulatory protein required for ribosome biogenesis in Saccharomyces cerevisiae.. Mol Cell Biol 2000; 20:2066 - 74; http://dx.doi.org/10.1128/MCB.20.6.2066-2074.2000; PMID: 10688653
- Zhang J, Harnpicharnchai P, Jakovljevic J, Tang L, Guo Y, Oeffinger M, et al. Assembly factors Rpf2 and Rrs1 recruit 5S rRNA and ribosomal proteins rpL5 and rpL11 into nascent ribosomes. Genes Dev 2007; 21:2580 - 92; http://dx.doi.org/10.1101/gad.1569307; PMID: 17938242
- Nariai M, Tanaka T, Okada T, Shirai C, Horigome C, Mizuta K. Synergistic defect in 60S ribosomal subunit assembly caused by a mutation of Rrs1p, a ribosomal protein L11-binding protein, and 3'-extension of 5S rRNA in Saccharomyces cerevisiae.. Nucleic Acids Res 2005; 33:4553 - 62; http://dx.doi.org/10.1093/nar/gki772; PMID: 16100378
- Morita D, Miyoshi K, Matsui Y, Toh-e A, Shinkawa H, Miyakawa T, et al. Rpf2p, an evolutionarily conserved protein, interacts with ribosomal protein L11 and is essential for the processing of 27 SB Pre-rRNA to 25 S rRNA and the 60 S ribosomal subunit assembly in Saccharomyces cerevisiae. J Biol Chem 2002; 277:28780 - 6; http://dx.doi.org/10.1074/jbc.M203399200; PMID: 12048200
- Huber MD, Dworet JH, Shire K, Frappier L, McAlear MA. The budding yeast homolog of the human EBNA1-binding protein 2 (Ebp2p) is an essential nucleolar protein required for pre-rRNA processing. J Biol Chem 2000; 275:28764 - 73; http://dx.doi.org/10.1074/jbc.M000594200; PMID: 10849420
- Horigome C, Okada T, Shimazu K, Gasser SM, Mizuta K. Ribosome biogenesis factors bind a nuclear envelope SUN domain protein to cluster yeast telomeres. EMBO J 2011; 30:3799 - 811; http://dx.doi.org/10.1038/emboj.2011.267; PMID: 21822217
- Miyoshi K, Shirai C, Horigome C, Takenami K, Kawasaki J, Mizuta K. Rrs1p, a ribosomal protein L11-binding protein, is required for nuclear export of the 60S pre-ribosomal subunit in Saccharomyces cerevisiae. FEBS Lett 2004; 565:106 - 10; http://dx.doi.org/10.1016/j.febslet.2004.03.087; PMID: 15135061
- Horigome C, Okada T, Matsuki K, Mizuta K. A ribosome assembly factor Ebp2p, the yeast homologue of EBNA1-binding protein 2, is involved in the secretory response. Biosci Biotechnol Biochem 2008; 72:1080 - 6; http://dx.doi.org/10.1271/bbb.70817; PMID: 18391458
- Miki F, Kurabayashi A, Tange Y, Okazaki K, Shimanuki M, Niwa O. Two-hybrid search for proteins that interact with Sad1 and Kms1, two membrane-bound components of the spindle pole body in fission yeast. Mol Genet Genomics 2004; 270:449 - 61; http://dx.doi.org/10.1007/s00438-003-0938-8; PMID: 14655046
- Starr DA, Fridolfsson HN. Interactions between nuclei and the cytoskeleton are mediated by SUN-KASH nuclear-envelope bridges. Annu Rev Cell Dev Biol 2010; 26:421 - 44; http://dx.doi.org/10.1146/annurev-cellbio-100109-104037; PMID: 20507227
- Jaspersen SL, Martin AE, Glazko G, Giddings TH Jr., Morgan G, Mushegian A, et al. The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J Cell Biol 2006; 174:665 - 75; http://dx.doi.org/10.1083/jcb.200601062; PMID: 16923827
- Schober H, Ferreira H, Kalck V, Gehlen LR, Gasser SM. Yeast telomerase and the SUN domain protein Mps3 anchor telomeres and repress subtelomeric recombination. Genes Dev 2009; 23:928 - 38; http://dx.doi.org/10.1101/gad.1787509; PMID: 19390087
- Dahl KN, Scaffidi P, Islam MF, Yodh AG, Wilson KL, Misteli T. Distinct structural and mechanical properties of the nuclear lamina in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA 2006; 103:10271 - 6; http://dx.doi.org/10.1073/pnas.0601058103; PMID: 16801550
- Dauer WT, Worman HJ. The nuclear envelope as a signaling node in development and disease. Dev Cell 2009; 17:626 - 38; http://dx.doi.org/10.1016/j.devcel.2009.10.016; PMID: 19922868
- Hediger F, Neumann FR, Van Houwe G, Dubrana K, Gasser SM. Live imaging of telomeres: yKu and Sir proteins define redundant telomere-anchoring pathways in yeast. Curr Biol 2002; 12:2076 - 89; http://dx.doi.org/10.1016/S0960-9822(02)01338-6; PMID: 12498682
- Taddei A, Hediger F, Neumann FR, Bauer C, Gasser SM. Separation of silencing from perinuclear anchoring functions in yeast Ku80, Sir4 and Esc1 proteins. EMBO J 2004; 23:1301 - 12; http://dx.doi.org/10.1038/sj.emboj.7600144; PMID: 15014445
- Ferreira HC, Luke B, Schober H, Kalck V, Lingner J, Gasser SM. The PIAS homologue Siz2 regulates perinuclear telomere position and telomerase activity in budding yeast. Nat Cell Biol 2011; 13:867 - 74; http://dx.doi.org/10.1038/ncb2263; PMID: 21666682
- Ebrahimi H, Donaldson AD. Release of yeast telomeres from the nuclear periphery is triggered by replication and maintained by suppression of Ku-mediated anchoring. Genes Dev 2008; 22:3363 - 74; http://dx.doi.org/10.1101/gad.486208; PMID: 19056887
- Hiraga S, Botsios S, Donaldson AD. Histone H3 lysine 56 acetylation by Rtt109 is crucial for chromosome positioning. J Cell Biol 2008; 183:641 - 51; http://dx.doi.org/10.1083/jcb.200806065; PMID: 19001125
- Ruault M, De Meyer A, Loïodice I, Taddei A. Clustering heterochromatin: Sir3 promotes telomere clustering independently of silencing in yeast. J Cell Biol 2011; 192:417 - 31; http://dx.doi.org/10.1083/jcb.201008007; PMID: 21300849
- Nagai S, Dubrana K, Tsai-Pflugfelder M, Davidson MB, Roberts TM, Brown GW, et al. Functional targeting of DNA damage to a nuclear pore-associated SUMO-dependent ubiquitin ligase. Science 2008; 322:597 - 602; http://dx.doi.org/10.1126/science.1162790; PMID: 18948542
- Kalocsay M, Hiller NJ, Jentsch S. Chromosome-wide Rad51 spreading and SUMO-H2A.Z-dependent chromosome fixation in response to a persistent DNA double-strand break. Mol Cell 2009; 33:335 - 43; http://dx.doi.org/10.1016/j.molcel.2009.01.016; PMID: 19217407
- Oza P, Jaspersen SL, Miele A, Dekker J, Peterson CL. Mechanisms that regulate localization of a DNA double-strand break to the nuclear periphery. Genes Dev 2009; 23:912 - 27; http://dx.doi.org/10.1101/gad.1782209; PMID: 19390086
- Oza P, Peterson CL. Opening the DNA repair toolbox: localization of DNA double strand breaks to the nuclear periphery. Cell Cycle 2010; 9:43 - 9; http://dx.doi.org/10.4161/cc.9.1.10317; PMID: 20016273
- Shirai C, Mizuta K. SUMO mediates interaction of Ebp2p, the yeast homolog of Epstein-Barr virus nuclear antigen 1-binding protein 2, with a RING finger protein Ris1p. Biosci Biotechnol Biochem 2008; 72:1881 - 6; http://dx.doi.org/10.1271/bbb.80131; PMID: 18603780
- Panse VG, Kressler D, Pauli A, Petfalski E, Gnädig M, Tollervey D, et al. Formation and nuclear export of preribosomes are functionally linked to the small-ubiquitin-related modifier pathway. Traffic 2006; 7:1311 - 21; http://dx.doi.org/10.1111/j.1600-0854.2006.00471.x; PMID: 16978391
- Mullen JR, Chen CF, Brill SJ. Wss1 is a SUMO-dependent isopeptidase that interacts genetically with the Slx5-Slx8 SUMO-targeted ubiquitin ligase. Mol Cell Biol 2010; 30:3737 - 48; http://dx.doi.org/10.1128/MCB.01649-09; PMID: 20516210
- Mullen JR, Das M, Brill SJ. Genetic evidence that polysumoylation bypasses the need for a SUMO-targeted Ub ligase. Genetics 2011; 187:73 - 87; http://dx.doi.org/10.1534/genetics.110.124347; PMID: 21059884
- Scherl A, Couté Y, Déon C, Callé A, Kindbeiter K, Sanchez JC, et al. Functional proteomic analysis of human nucleolus. Mol Biol Cell 2002; 13:4100 - 9; http://dx.doi.org/10.1091/mbc.E02-05-0271; PMID: 12429849
- Gambe AE, Matsunaga S, Takata H, Ono-Maniwa R, Baba A, Uchiyama S, et al. A nucleolar protein RRS1 contributes to chromosome congression. FEBS Lett 2009; 583:1951 - 6; http://dx.doi.org/10.1016/j.febslet.2009.05.033; PMID: 19465021
- Romanova L, Grand A, Zhang L, Rayner S, Katoku-Kikyo N, Kellner S, et al. Critical role of nucleostemin in pre-rRNA processing. J Biol Chem 2009; 284:4968 - 77; http://dx.doi.org/10.1074/jbc.M804594200; PMID: 19106111
- Kapoor P, Frappier L. EBNA1 partitions Epstein-Barr virus plasmids in yeast cells by attaching to human EBNA1-binding protein 2 on mitotic chromosomes. J Virol 2003; 77:6946 - 56; http://dx.doi.org/10.1128/JVI.77.12.6946-6956.2003; PMID: 12768013
- Nayyar VK, Shire K, Frappier L. Mitotic chromosome interactions of Epstein-Barr nuclear antigen 1 (EBNA1) and human EBNA1-binding protein 2 (EBP2). J Cell Sci 2009; 122:4341 - 50; http://dx.doi.org/10.1242/jcs.060913; PMID: 19887584
- Wang Q, Du X, Cai Z, Greene MI. Characterization of the structures involved in localization of the SUN proteins to the nuclear envelope and the centrosome. DNA Cell Biol 2006; 25:554 - 62; http://dx.doi.org/10.1089/dna.2006.25.554; PMID: 17132086
- Chi YH, Haller K, Peloponese JM Jr., Jeang KT. Histone acetyltransferase hALP and nuclear membrane protein hsSUN1 function in de-condensation of mitotic chromosomes. J Biol Chem 2007; 282:27447 - 58; http://dx.doi.org/10.1074/jbc.M703098200; PMID: 17631499
- Hirano Y, Ishii K, Kumeta M, Furukawa K, Takeyasu K, Horigome T. Proteomic and targeted analytical identification of BXDC1 and EBNA1BP2 as dynamic scaffold proteins in the nucleolus. Genes Cells 2009; 14:155 - 66; http://dx.doi.org/10.1111/j.1365-2443.2008.01262.x; PMID: 19170763
- Gasser SM, Amati BB, Cardenas ME, Hofmann JF. Studies on scaffold attachment sites and their relation to genome function. Int Rev Cytol 1989; 119:57 - 96; http://dx.doi.org/10.1016/S0074-7696(08)60649-X; PMID: 2695485
- Fey EG, Krochmalnic G, Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol 1986; 102:1654 - 65; http://dx.doi.org/10.1083/jcb.102.5.1654; PMID: 3700470
- Parfenov VN, Davis DS, Pochukalina GN, Sample CE, Bugaeva EA, Murti KG. Nuclear actin filaments and their topological changes in frog oocytes. Exp Cell Res 1995; 217:385 - 94; http://dx.doi.org/10.1006/excr.1995.1101; PMID: 7698240
- Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, Wilson KL. Actin- and protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci 2004; 117:2481 - 90; http://dx.doi.org/10.1242/jcs.01098; PMID: 15128868
- Martin C, Chen S, Maya-Mendoza A, Lovric J, Sims PF, Jackson DA. Lamin B1 maintains the functional plasticity of nucleoli. J Cell Sci 2009; 122:1551 - 62; http://dx.doi.org/10.1242/jcs.046284; PMID: 19383719
- Mekhail K, Seebacher J, Gygi SP, Moazed D. Role for perinuclear chromosome tethering in maintenance of genome stability. Nature 2008; 456:667 - 70; http://dx.doi.org/10.1038/nature07460; PMID: 18997772
- Grund SE, Fischer T, Cabal GG, Antúnez O, Pérez-Ortín JE, Hurt E. The inner nuclear membrane protein Src1 associates with subtelomeric genes and alters their regulated gene expression. J Cell Biol 2008; 182:897 - 910; http://dx.doi.org/10.1083/jcb.200803098; PMID: 18762579