Abstract
Novel, potent tumor-associated antigens are needed to improve the efficacy of immunotherapy for myeloma. We demonstrated that active vaccination using the DKK1-DNA vaccine in the myeloma mouse model protected mice from developing myeloma and effectively treated established myeloma. Therefore, DKK1 could be developed as a novel vaccine for myeloma immunotherapy.
Multiple myeloma (MM) is a plasma cell malignancy that remains incurable in the vast majority of patients. Unlike other malignancies, MM cells express only a few identified tumor-associated antigens, only one of which, the idiotypic monoclonal immunoglobulin (Ig), has been tested in clinical trials. We and others have explored immunotherapy using idiotype-based vaccines for myeloma, but those studies have yielded disappointing results.Citation1,Citation2 A partial explanation for the inefficacy of these idiotype-based vaccines is the weak immunogenicity of idiotype proteins and the clonal exhaustion and deletion of idiotype-specific T cells in patients due to the presence of large amounts of circulating idiotype protein via antigen-presenting cells and/or MM cells.Citation3,Citation4 Therefore, novel and more potent tumor-associated antigens, especially those shared among patients, must be identified to improve the efficacy of immunotherapy for this disease.
Recent studies have shown that Dickkopf-1 (DKK1), a secreted protein and Wnt signaling pathway inhibitor, is highly expressed by the tumor cells of almost all patients with MMCitation5 and is absent from normal tissues and organs, except placenta and prostate.Citation6 Furthermore, MM cell-derived DKK1 may be responsible, at least in part, for suppressed osteoblast formation and bone destruction associated with MM,Citation5 and inhibiting DKK1 activity increased osteoblast activity and bone formation, reduced osteoclast activity, and inhibited MM growth in an MM animal model.Citation7 Our previous results clearly showed that DKK1 is expressed by the primary tumor cells of all patients with MM, and DKK1 (peptide)-specific cytotoxic T lymphocytes (CTLs) can effectively lyse primary MM cells in vitro.Citation8 Hence, we hypothesized that the broad expression in MM but highly restricted expression in normal tissues, together with its functional roles as an osteoblast formation inhibitor and a potential MM growth enhancer, render DKK1 an ideal and universal target for immunotherapy in MM.
The goal of our recent studyCitation9 was to determine whether DKK1 could be used as a tumor vaccine to elicit DKK1-specific immunity for controlling MM growth or even eradicating established MM in vivo. Therefore, we designed the expression plasmid encoding murine DKK1 cDNA-defensin-2 fusion gene and tested it in the MOPC-21 MM mouse model. In prophylactic studies, Balb/c mice (10 per group) were immunized with the DNA (murine DKK1/defensin-2 fusion) vaccine before tumor challenge. All the mice in groups that received injections of phosphate-buffered saline (PBS) or vector vaccine developed tumors. However, the survival rates of mice that received the DKK1-DNA vaccine alone or supplemented with CpG were 30% and 60%, respectively, by end of the experiment (day 90: p < 0.05 for DKK1-DNA vaccine and p < 0.01 for DKK1-DNA vaccine plus CpG, compared with PBS control). Our results showed that the DKK1-DNA vaccine plus adjuvant CpG generated superior protection against tumor challenge.
Next, we evaluated the therapeutic efficacy of the DKK1-DNA vaccine in mice with established MM using the DKK1-DNA vaccine plus CpG as a standard vaccine. In a previous study, we found that targeting the suppressive tumor microenvironment and interrupting T-cell immunosuppression increased the immunogenicity of the vaccines and cured mice with large tumor burdens.Citation10 Therefore, to optimize the therapeutic efficacy of DKK1 vaccination, we combined the DKK1 vaccine plus CpG with anti-B7H1 (M5H1) monoclonal antibody (mAb) to block negative T-cell signaling or an agonist anti-OX40 (OX86) mAb to overcome CD4+ T-cell tolerance. Mice that received the DKK1-DNA vaccine plus CpG had less tumor growth than control mice (p < 0.05). The DKK1-DNA vaccine plus CpG combined with the B7H1 or OX40 mAb inhibited tumor growth to an even greater extent than the DKK1-DNA vaccine plus CpG (p < 0.01, compared with rat IgG control mice). Thus, the DKK1-DNA vaccine was effective for treating MM in the murine model with large established tumors, and B7H1-blocking or OX40 agonist mAbs augmented the therapeutic efficacy of the vaccine.
Our immunologic studies showed that the vaccine elicited strong DKK1- and MM-specific CD4+ and CD8+ interferon-gamma T-cell and CTL responses, and the depletion of CD4+ and especially CD8+ T cells in vivo abrogated the anti-tumor effects of the vaccine. In vivo injections of CpG or B7H1-blocking or OX40-agonist mAb amplified the effectiveness of the vaccine by increasing the frequency of DKK1- and MM-specific effector (interferon-gamma-secreting and CTL) T-cells and decreasing the numbers of interleukin-10-secreting and Foxp3+ Treg cells. shows the schema of anti-tumor immunity following DNA vaccination with DKK1/defensin-2 DNA fusion vaccine.
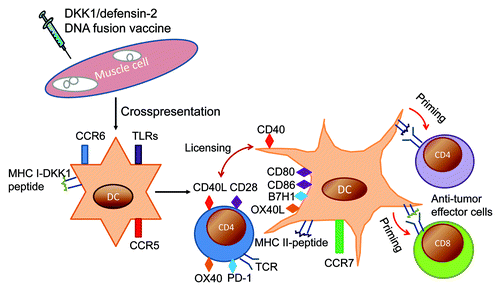
Figure 1. Proposed schema of anti-tumor immunity following DNA vaccination with DKK1/defensin-2 DNA fusion vaccine. Following intramuscular injection in mice of DKK1/defensin-2 DNA fusion vaccine, muscle and resident APCs are transfected with plasmid, leading to fusion protein production. Murine defensin-2 plays a role in activating APCs via CCL-6 and TLR4. Cross-priming occurs in which CD8+ T cell responses are primed by exogenous MHC Class I–restricted antigens that are not expressed in, but rather are acquired by, local APCs. These DCs (dendritic cells) acquire antigen, express CCR7, and are attracted by chemokines expressed in the draining lymph nodes, where they prime naive T cells. DKK1 is processed, and peptides are presented by MHC Class I molecules; this peptide/MHC Class I complex stimulates CD8+ T lymphocytes. Soluble protein released by transfected cells is taken up by DCs, and via the MHC Class II pathway, the peptide/MHC Class II complex stimulates CD4+ T lymphocytes, and MHC Class II-binding peptides from DKK1 activate the large repertoire of anti-DKK1 CD4+ T cells. Pairs of receptor–ligands (matched colors) interact to 'license' the DCs to maintain presentation of tumor-derived peptides that are able to prime anti-tumor CD8+ and CD4+ T cells. CpGs interact with TLR9 in APCs, where they induce the expression of costimulatory molecules such as CD80 and CD86, MHC Class II molecules, and pro-inflammatory cytokines. B7H1-blocking antibodies interrupt T-cell immunosuppression through PD-1/B7 family signaling. OX40-agonist antibodies might affect the roles of Treg cells through OX40 signaling since mouse Treg cells constitutively express OX40. Therefore, DKK1 DNA vaccine plus CpG combined with B7H1-blocking or OX40-agonist antibodies could break the immunosuppression of T-cell response and lead to much better anti-tumor immunity induced by the vaccine. TCR, T-cell receptor.
These findings strongly suggest that DKK1 can be used as a universal tumor vaccine for active immunotherapy of MM and provide a rationale and platform for future clinical application of DKK1-based immunotherapy in patients with MM. However, for future clinical application, administration of the DKK1 vaccine as recombinant protein or (short and long) peptides might be more suitable than DNA (plasmid). Future work is needed to determine which form of the vaccine works best.
| Abbreviations: | ||
| DKK1 | = | Dickkopf-1 |
| MM | = | multiple myeloma |
| Ig | = | immunoglobulin |
| CTLs | = | cytotoxic T lymphocytes |
| mAb | = | monoclonal antibody |
| PBS | = | phosphate-buffered saline |
References
- Titzer S, Christensen O, Manzke O, Tesch H, Wolf J, Emmerich B, et al. Vaccination of multiple myeloma patients with idiotype-pulsed dendritic cells: immunological and clinical aspects. Br J Haematol 2000; 108:805 - 16; http://dx.doi.org/10.1046/j.1365-2141.2000.01958.x; PMID: 10792287
- Osterborg A, Yi Q, Henriksson L, Fagerberg J, Bergenbrant S, Jeddi-Tehrani M, et al. Idiotype immunization combined with granulocyte-macrophage colony-stimulating factor in myeloma patients induced type I, major histocompatibility complex-restricted, CD8- and CD4-specific T-cell responses. Blood 1998; 91:2459 - 66; PMID: 9516146
- Yi Q, Desikan R, Barlogie B, Munshi N. Optimizing dendritic cell-based immunotherapy in multiple myeloma. Br J Haematol 2002; 117:297 - 305; http://dx.doi.org/10.1046/j.1365-2141.2002.03411.x; PMID: 11972511
- Yi Q. Dendritic cell-based immunotherapy in multiple myeloma. Leuk Lymphoma 2003; 44:2031 - 8; http://dx.doi.org/10.1080/1042819031000116599; PMID: 14959845
- Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B, et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. [see comment] N Engl J Med 2003; 349:2483 - 94; http://dx.doi.org/10.1056/NEJMoa030847; PMID: 14695408
- Krupnik VE, Sharp JD, Jiang C, Robison K, Chickering TW, Amaravadi L, et al. Functional and structural diversity of the human Dickkopf gene family. Gene 1999; 238:301 - 13; http://dx.doi.org/10.1016/S0378-1119(99)00365-0; PMID: 10570958
- Yaccoby S, Ling W, Saha R, Zhan F, Walker R, Tricot G, et al. Inhibition of DKK1 activity is associated with increased osteoblast numbers and bone formation, reduced osteoclast activity and inhibtion of tumor growth in the SCID-rab model for primary myeloma. Blood 2005; 106:189a
- Qian J, Xie J, Hong S, Yang J, Zhang L, Han X, et al. Dickkopf-1 (DKK1) is a widely expressed and potent tumor-associated antigen in multiple myeloma. Blood 2007; 110:1587 - 94; http://dx.doi.org/10.1182/blood-2007-03-082529; PMID: 17515399
- Qian J, Zheng Y, Zheng C, Wang L, Qin H, Hong S, et al. Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood 2012; 119:161 - 9; http://dx.doi.org/10.1182/blood-2011-07-368472; PMID: 22049519
- Qian J, Hong S, Wang S, Zhang L, Sun L, Wang M, et al. Myeloma cell line-derived, pooled heat shock proteins as a universal vaccine for immunotherapy of multiple myeloma. Blood 2009; 114:3880 - 9; http://dx.doi.org/10.1182/blood-2009-06-227355; PMID: 19654406