Abstract
Regulatory T cells (Treg) have been reported of poor prognosis for overall survival in primary breast tumors (BT). As CCL22 plays a major role in Treg recruitment within primary BT we deciphered the mechanisms involved in the CCL22 production by breast epithelial tumor cells and propose herein the major role of their innate immune recognition in this production.
Breast cancer is an immunogenic tumor as (1) CD8+ T cell response as well as humoral responses against Tumor associated Antigens (TAA) (Her2neu, p53, Muc1) have been demonstrated and (2) CD8+ T cell infiltration has been recently reported to correlate with better prognosis.Citation1 Moreover we recently demonstrated that primary breast tumors (BT) are largely infiltrated by immune cells involved in innate sensing i.e., NK cells, dendritic cells (DC) and macrophages (MΦ) that present an activated phenotypeCitation2,Citation3 suggesting their stimulation within the BT environment. We also recently reported the presence of strong CD4+ T lymphocytes infiltrates in BTCitation4 suggesting that all the players required to set-up an efficient anti-tumor response are present within the BT environment.
However, when tumors are clinically detected, this immune response is, in most cases, unable to counteract cancer development because tumors have developed immuno-subversion processes. Several studies including from our group demonstrated the infiltration of BT by immune subsets involved in immune tolerance i.e., plasmacytoïd DCCitation3 and CD4+CD25highCD127negFoxP3+ regulatory T cells (Treg)Citation1,Citation4 and Type 2 MΦ (for review see ref. Citation5) that are all of poor prognosis for overall survival (OS) in primary BT.
An in-depth ex vivo analysis demonstrate that tumor associated Treg (TA-Treg) (1) are activated as they express ICOS, HLA-DR and CTLA-4, (2) are functional as they suppress CD4+ T cells proliferation and IFNγ secretion, (3) promote immune-suppressive environment by favoring IL-10 secretion and (4) proliferate in situ in contrast to the resting non regulatory CD4+ memory T cells and CD8+ T cells detected within BT.Citation4
In contrast to associated patients’ blood Treg, TA-Treg present a selective loss of membrane CCR4, consecutive to an active recruitment through CCL22 secreted within the BT environment. In line with this, BT lacking CCL22 are not infiltrated by TA-Treg independently of their production of CCL17, the other CCR4 ligand. Of note CCL22, but not CCL17 induced the CCR4 downregulation.Citation4
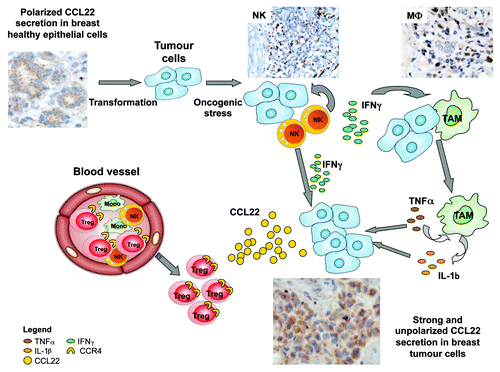
CCL22 has been reported to be secreted by myeloïd DC, B cells, MΦ, or epithelial cells, all subsets detectable within the BT environment. As previously described for other chemokines (GRO-α/β/γ, CXCL8, MIG, IP-10 and RANTES) we have demonstrated by immuno-histochemistry (IHC), in peri-tumoral breast tissue, a polarized apical secretion of CCL22 by healthy luminal epithelial cells within lobular aciniCitation2 () suggesting CCL22 is part of the mammary gland physiology to control local inflammation associated with menstrual cycle or breastfeeding.
Figure 1. Scheme recapitulating the sequence of events leading to the strong non polarized CCL22 production by tumor cells. Healthy epithelial cells secrete low levels of CCL22 in a polarized manner within the luminal acini, their transformation favor their recognition by infiltrating NK cells leading to IFNγ secretion. IFNγ promoted macrophage activation that will produce TNFα and IL-1β after interaction with breast epithelial tumor cells. Combined action of IFNγ, IL-1β and TNFα will induce non polarized strong CCL22 secretion by tumor cells that will induce the recruitment of CCR4+ Treg from periphery, leading to CCR4 internalization.
In primary BT context, independently of the molecular subtype of the tumor, CCL22 expression is strongly increased as assessed by IHC but also by ELISA within the BT dilacerations supernatants.
Interestingly, at the systemic level, we could observe a gradual increase in CCL22 plasmatic levels from healthy subjects, patients with primary BT, 1st metastatic relapse or with more advanced BT (Tredan O., manuscript in preparation) that could reflect the tumor burden.
Using BT epithelial cell lines but also primary BT specimens, we recently demonstrated the major role of immune infiltrate in the selective induction of CCL22 but not CCL17 by tumor epithelial cells.Citation2 In-depth analyses through in vitro experiments using (1) inhibitory antibodies against cytokine receptors and/or cytokines or (2) addition of exogenous recombinant cytokines, demonstrate the preponderant role of a dialog between tumor epithelial cells, infiltrating NK cells and MΦ for this CCL22 production.Citation2 Through these studies we propose the following sequence of events (): (1) NK cells detecting tumor cells secrete IFNγ, (2) IFNγ activates MΦ favoring their secretion of IL-1β and TNFα, (3) these cytokines act together to increase CCL22 production by epithelial tumor cells. This was further confirmed in ex vivo experiments using primary BT specimens demonstrating the cooperation of MΦ and NK cells to favor CCL22 production by freshly purified tumor cells.
This illustrates a mechanism allowing the breast transformed epithelial cells to counteract the local inflammation involving NK and MΦ to favor Treg recruitment through CCL22 secretion as previously described in chronically inflamed colon.Citation6 In turn, TA-Treg may also favor tumor progression via (1) the inhibition of NK cytolytic functions (for review see ref. Citation7), (2) the conversion, as recently demonstrated in HIV context,Citation8 of Type 1 MΦ into Type 2 MΦ that have pro-tumor functions through production of factors promoting angiogenesis, tumor cell proliferation and favoring immunosuppression (for review see ref. Citation5).
Apart its function in immune cell recruitment, CCL22 has recently been described, in a murine model, to reduce the Ag specific proliferation of CD4+ T cells.Citation9 In this context, high CCL22 production within the tumor environment may favor the blockade of TAA-specific CD4+ T cell proliferation.
All together, these data strongly indicate that CCL22 participate to immuno-subversion in BT and will favor disease progression. In this context, CCR4 antagonists have been validated to block in vitro CCL22-mediated recruitment of human Treg and Th2 cells and favored a break of peripheral tolerance to self antigen in murine model by blocking Treg migration.Citation10 However, selective CCL22 antagonists rather than CCR4 that will not target CCL17 would represent a more selective target to prevent Treg recruitment within tumors.
References
- Liu F, Lang R, Zhao J, Zhang X, Pringle GA, Fan Y, et al. CD8⁺ cytotoxic T cell and FOXP3⁺ regulatory T cell infiltration in relation to breast cancer survival and molecular subtypes. Breast Cancer Res Treat 2011; 130:645 - 55; http://dx.doi.org/10.1007/s10549-011-1647-3; PMID: 21717105
- Faget J, Biota C, Bachelot T, Gobert M, Treilleux I, Goutagny N, et al. Early detection of tumor cells by innate immune cells leads to T(reg) recruitment through CCL22 production by tumor cells. Cancer Res 2011; 71:6143 - 52; http://dx.doi.org/10.1158/0008-5472.CAN-11-0573; PMID: 21852386
- Treilleux I, Blay JY, Bendriss-Vermare N, Ray-Coquard I, Bachelot T, Guastalla JP, et al. Dendritic cell infiltration and prognosis of early stage breast cancer. Clin Cancer Res 2004; 10:7466 - 74; http://dx.doi.org/10.1158/1078-0432.CCR-04-0684; PMID: 15569976
- Gobert M, Treilleux I, Bendriss-Vermare N, Bachelot T, Goddard-Leon S, Arfi V, et al. Regulatory T cells recruited through CCL22/CCR4 are selectively activated in lymphoid infiltrates surrounding primary breast tumors and lead to an adverse clinical outcome. Cancer Res 2009; 69:2000 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-08-2360; PMID: 19244125
- Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol 2010; 22:231 - 7; http://dx.doi.org/10.1016/j.coi.2010.01.009; PMID: 20144856
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annu Rev Immunol 2009; 27:313 - 38; http://dx.doi.org/10.1146/annurev.immunol.021908.132657; PMID: 19302043
- Ralainirina N, Poli A, Michel T, Poos L, Andrès E, Hentges F, et al. Control of NK cell functions by CD4+CD25+ regulatory T cells. J Leukoc Biol 2007; 81:144 - 53; http://dx.doi.org/10.1189/jlb.0606409; PMID: 16959895
- Huang X, Stone DK, Yu F, Zeng Y, Gendelman HE. Functional proteomic analysis for regulatory T cell surveillance of the HIV-1-infected macrophage. J Proteome Res 2010; 9:6759 - 73; http://dx.doi.org/10.1021/pr1009178; PMID: 20954747
- Beaty SR, Rose CE Jr., Sung SS. Diverse and potent chemokine production by lung CD11bhigh dendritic cells in homeostasis and in allergic lung inflammation. J Immunol 2007; 178:1882 - 95; PMID: 17237439
- Pere H, Montier Y, Bayry J, Quintin-Colonna F, Merillon N, Dransart E, et al. A CCR4 antagonist combined with vaccines induces antigen-specific CD8+ T cells and tumor immunity against self antigens. Blood 2011; 118:4853 - 62; http://dx.doi.org/10.1182/blood-2011-01-329656; PMID: 21908423