Abstract
An important development in tumor immunology was the identification of highly diverse tumor-infiltrating leukocyte subsets that can play strikingly antagonistic functions. Namely, “anti-tumor” vs. “pro-tumor” roles have been suggested for Th1 and Th17 subsets of CD4+ T cells, Type I or Type II NKT cells, M1 and M2 macrophages, or N1 and N2 neutrophils, respectively. While these findings are being validated in cancer patients, it is also clear that the balance between infiltrating CD8+ cytotoxic and Foxp3+ regulatory T cells has prognostic value. Here we review the pre-clinical and clinical data that have shaped our current understanding of tumor-infiltrating leukocytes.
Introduction
A fundamental principle of cancer immune surveillance is that tumors are infiltrated by leukocytes, particularly lymphocytes, capable of recognizing and targeting transformed cells, thus leading to their elimination before the tumor becomes clinically apparent. Moreover, the efficacy of immunotherapy against established tumors presumably depends on lymphocyte recruitment and effector function within the tumor bed. However, a major obstacle to anti-cancer therapy is the local immune suppression commonly found within the tumor microenvironment.Citation1 While earlier work had focused on tumor cell-derived factors that inhibit the local immune response, the past few years have demonstrated a dramatic contribution of leukocytes themselves to this “pro-tumor” environment. Recent reports have further clarified this paradoxical leukocyte behavior by identifying a very heterogeneous set of subpopulations, both of lymphoid and myeloid origin, that can play strikingly antagonistic roles within the tumors they co-infiltrate.
The prototypic anti-tumor function, displayed by various lymphocyte subsets (), is cytotoxicity via the perforin/ granzyme system or, alternatively, by engaging death receptors (such as Fas). These properties are further promoted by interferon γ (IFNγ), the signature Th1 cytokine that is, in fact, secreted by multiple cell types (see below), often together with tumor necrosis factor (TNF). By contrast, cytokines such as TGFβ or IL-10 are highly immunosuppressive, and other secreted factors, like VEGF, directly promote angiogenesis and thus tumor growth (). The detailed characterization of gene expression and cytokine profiles in leukocyte populations isolated from tumor biopsies (or draining lymph nodes) has been instrumental in revealing the heterogeneity of tumor-infiltrating leukocytes, both of lymphoid and myeloid nature, which we will discuss in this review.
Figure 1. Anti-tumor infiltrating leukocytes and molecular mechanisms of action. Representation of the main anti-tumor lymphoid and myeloid cells. N1 and M1 refer to neutrophil and macrophage subsets, respectively. γδ1 and Th1 refer to IFNγ-producing γδ and CD4+ T cells, respectively. Depicted are also molecules produced by these leukocytes, including cytokines that impact on cell differentiation and expansion, and chemokines that control their recruitment/infiltration into tumors.
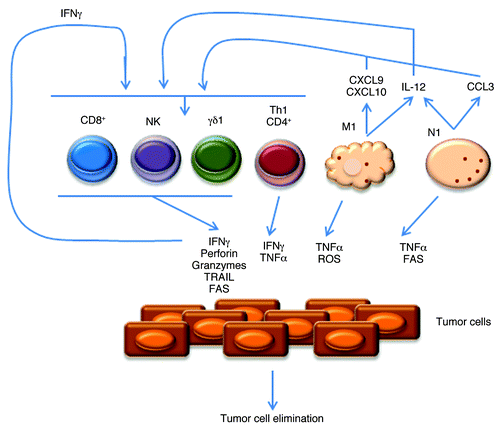
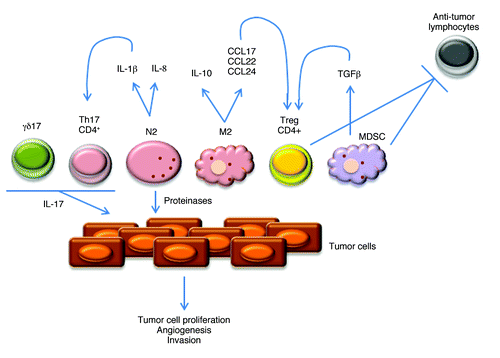
Figure 2. Pro-tumor infiltrating leukocytes and molecular mechanisms of action. Representation of the main pro-tumor lymphoid and myeloid cells. N2 and M2 refer to neutrophil and macrophage subsets, respectively. γδ17 and Th17 refer to IL-17-producing γδ and CD4+ T cells, respectively. Depicted are also molecules produced by these leukocytes, including cytokines that impact on cell differentiation and expansion, and chemokines that control their recruitment/infiltration into tumors.
Many of the key studies on tumor-infiltrating leukocytes have been performed in mouse tumor models. Although they present several important limitations, including the artificial homogeneity and laboratory selection of tumor cell lines used in transplantable models, the lack of relevant physiology (including interactions between autologous tumors and immune cells) in xenograft models, and the commonly short span (2–4 weeks) of all these tumor development experiments, animal models provide a unique possibility of tracking and manipulating cancerigenesis in vivo.
This notwithstanding, it is obviously essential to validate all the findings from mouse tumor models in human cancer samples. Therefore, in this review we will discuss and summarize the most recent advances, both in the laboratory and in the clinic, in our understanding of the biology of tumor-infiltrating leukocytes. We will highlight their anti- or pro-tumor functions in mouse models, and how these translate (or not) into prognostic value in cancer patients.
The Traditional Players: NK, CD8+ T and Th1 Cells
It has been known for three decades that NK cells and CD8+ T lymphocytes, including those extracted from tumor biopsies, can efficiently kill transformed cells. Collectively, these killer lymphocytes recognize two important types of tumor antigens (among others): processed peptides presented by MHC Class Ia proteins via TCRαβ; and non-classical (Class Ib) MHC proteins via NKG2D.Citation2 The latter, which is expressed on NK, CD8+ and also γδ T cells, has been recently shown to be a key genetic determinant of cancer immune surveillance.Citation3
NK and CD8+ cells provide highly complementary anti-tumor strategies. Indeed, as demonstrated by the seminal work of Kärre and Kiessling, the downregulation of MHC class Ia, which is a common mechanism of evasion against CD8+ cells, renders tumors more susceptible to NK cell-mediated lysis. This “missing self” recognition by NK cells is based on a set of MHC class Ia-specific inhibitory receptors that include killer cell immunoglobulin-like receptors (KIRs) in humans, lectin-like Ly49 molecules in mice, and CD94/NKG2A heterodimers in both species; in fact, NK cells express a complex repertoire of inhibitory and activating receptors that calibrate this anti-tumor function, while ensuring self-tolerance.Citation4,Citation5 In result, NK cells eliminate tumors that lack MHC class Ia expression; or that overexpress ligands for activating NK receptors like NKG2D or the natural cytotoxicity receptors NKp30, NKp44 and NKp46.Citation5 Furthermore, NK cells express high levels of low-affinity Fc receptor for IgG (CD16), which allows them to mediate antibody-dependent cell-mediated cytotoxicity (ADCC).Citation6
NK cells have been described to infiltrate various types of tumors in the skin, lung, gut and kidney.Citation5 Recent data on human NK cells infiltrating highly aggressive non-small cell lung cancers (NSCLC) showed a profound alteration of their phenotype, with decreased ability to degranulate and to produce IFNγ, when compared with NK cells from distal lung tissues or blood from the same patients or from healthy donors.Citation7 This functional impairment of NK-TILs correlated with decreased expression of NKp30, NKp80, DNAM-1, CD16 and ILT2 receptors. Interestingly, among these, NKp30 has been shown to affect the prognosis of gastrointestinal stromal tumors through a specific pattern of alternative splicing.Citation8
Various immunotherapeutic strategies have been proposed to tackle the common defects of NK cell activity in cancer patients:Citation5 activation of endogenous NK cells (with cytokines like IL-2, IL-15 and IL-18), NK-cell adoptive immunotherapy, NK-cell-based donor lymphocyte infusions and allogenic stem cell transplantation (SCT).Citation6 Although globally the objective responses have been disappointing, some data from allogenic and, more recently, haploidentical hematopoietic SCT have shown clinical (in the absence of adverse) effects mediated by NK cells.Citation5 This inspires further translational studies aimed at enhancing NK cell recruitment to tumors and their functional activity in situ.
With regard to CD8+ T cell-based immunotherapy, many recent efforts have focused in activating and expanding CD8+ tumor-infiltrating lymphocytes (TILs) ex vivo and then re-infusing them into the cancer patient—adoptive cell therapy (ACT). ACT of CD8+ TILs into lymphodepleted metastatic melanoma patients has shown very high objective response rates, ranging from 50% up to 81%.Citation9 In fact, TIL-ACT (combined with high doses of IL-2) has mediated cancer regression in 49–72% of melanoma patients, and durable complete responses, beyond 3–7 y, are currently ongoing in 40% of the patients.Citation10
In pre-clinical models, adoptively transferred naïve CD8+ cells were shown to infiltrate melanoma lesions, be activated in situ and differentiate into functional cytotoxic T lymphocytes (CTLs).Citation11 The naïve status of the infused population appeared to be an important parameter, as the differentiation stage of CTLs inversely correlated with their anti-tumor efficacy in vivo.Citation12 The enhanced anti-tumor function of naïve T cells was related to sustained effector cell development, prolonged cytokine production, and increased expansion in vivo.
Transduction of tumor antigen-specific TCRsCitation13 or chimeric antigen receptors (CARs)Citation14,Citation15 represent exciting prospects to increase the efficacy of cytotoxic ACT. These strategies have thus far enabled cancer regression in patients with metastatic melanoma, synovial sarcoma, neuroblastoma and refractory lymphoma or leukemia.Citation10
In addition to cytotoxicity, IFNγ secretion is a key anti-tumor function of CD8+ and NK cells, who share this property with various other lymphocyte populations, most notably “helper type 1” (Th1) CD4+ cells. These were first described 25 y ago in the context of the “Th1/ Th2” paradigm of immunity to infection, and since then clearly implicated in promoting anti-tumor responses: Th1 cells enhance the cytotoxic functions of NK and CD8+ cells, upregulate MHC Class I expression in tumor cells (a direct effect of IFNγ), and support CD8+ cell proliferation through the secretion of IL-2.Citation16 Moreover, Th1 cells condition the antigen-presenting capacity of DCs and macrophages, thus shaping the CTL response. In fact, the combination of Th1 cell therapy with local radiation therapy augmented the generation of tumor-specific CTL at the tumor site and induced a complete regression of subcutaneous tumors.Citation17
“New” Effector TILs: γδ T, NKT and Th17 Cells
The “Th1/Th2” paradigm for CD4+ T cell differentiation has been recently revised with the addition of Th17 cells, characterized by the production of interleukin-17 (IL-17). IL-17-deficient mice were shown to be more susceptible (than wild type animals) to tumor growth and lung metastasis.Citation18,Citation19 Adoptive transfer studies from the Restifo lab showed that in vitro generated Th17 cells were more efficient at eradicating tumors than Th1 cells,Citation20 and this was recently associated with stem cell-like properties of Th17 cells.Citation21 Importantly, adoptively transferred Th17 cells gave rise in vivo to Th1-like effector cell progeny,Citation21 and IFNγ was actually necessary for the protective effects of adoptively transferred Th17 cells.Citation20 These data suggest that acquisition of Th1-like properties are required for an anti-tumor function by Th17 cells.
In stark contrast to the previous studies, IL-17-deficient mice presented reduced tumor growth in other models such as B16 melanoma and MB49 bladder carcinoma,Citation22 DMBA/TPA-induced skin carcinoma,Citation23 or in a spontaneous intestinal tumor model (driven by a mutation in the tumor suppressor gene APC).Citation24
The pro-tumor functions of IL-17 have been tightly linked to angiogenesis: IL-17 has been shown to act on endothelial, stromal and tumor cells to induce the expression of pro-angiogenic factors like VEGF, Angiotensins, PGE2 and IL-8, and thus promote tumor vascularization.Citation25 The precise conditions that determine pro- vs. anti-tumor functions of Th17 TILs remain unclear and require further investigation.
Although Th17 cells are important providers of IL-17, this cytokine can be abundantly produced by other tumor-infiltrating leukocyte populations. Namely, murine γδ T cells can be the major source of IL-17, not only in homeostatic conditions,Citation26 but also upon infection or tumor challenge.Citation27,Citation28 Like for Th17 cells, the role of IL-17 produced by γδ cells within the tumor microenvironment is controversial: it has been associated both with angiogenesis and promotion of tumor growthCitation25,Citation27; and with CD8+ T cell recruitment and the therapeutic effects of chemotherapy against several subcutaneous tumor lines.Citation28,Citation29
While the recently discovered ability of γδ cells to make IL-17 has attracted much attention, these lymphocytes were previously characterized as strong cytotoxic and IFNγ-producing cells, and thus prototypic anti-tumor mediators. Consistent with this, seminal work by Girardi and Hayday showed a decade ago that mice lacking γδ T cells were significantly more susceptible to chemically induced tumors.Citation30 This phenotype was subsequently extended to transplantable,Citation31 spontaneousCitation32 and transgenicCitation33 tumors.
In the murine B16 melanoma model, γδ T cells were shown to infiltrate tumor lesions already at day 3 post-transplantation and to provide a critically early source of IFNγ.Citation31 This contrasts with the above-mentioned findings on IL-17+ γδ-TILs.Citation27,Citation28 A more detailed characterization of γδ-TILs is therefore required in a wider set of pre-clinical tumor models. This should take into account the two functional γδ T cell subsets recently identified on the basis of CD27 (and CCR6) expression: CD27+ γδ cells make IFNγ but no IL-17, whereas IL-17 production is restricted to CD27- γδ cells.Citation34
γδ T cell-based clinical trials have thus far concentrated on the highly IFNγ-polarized (and cytotoxic) Vγ9Vδ2 subset that constitutes most of γδ cells circulating in the human peripheral blood. As these cells are specifically reactive to non-peptidic phosphoantigens, they can be selectively activated and expanded both in vitro (for ACT) and in vivo. In cancer patients, γδ T cell-based immunotherapy has thus far produced objective responses in the range of 10 to 33%.Citation35 Future research should also take into account the important roles played by NK receptors, including NKG2DCitation36 and NKp30,Citation37 in tumor cell recognition by Vγ9Vδ2 cells and by Vδ1 cells (which predominate in tissues).
NKT cells also employ NK receptors, as well as CD1d-restricted TCRs to recognize tumor targets. The vast majority of these T cells are canonical or invariant NKT (type I NKT) cells that possess a specific TCRα rearrangement (Vα14Jα18 in mice; Vα24Jα18 in humans), associated with Vβ chains of limited diversity. All the other NKT cells that are CD1d-restricted and do not express this invariant TCR are called Type II NKT cells.Citation38,Citation39 Although CD1d-deficient mice showed increased susceptibility to MCA-induced sarcomas,Citation40 there is evidence of functional heterogeneity also within NKT cells: while Type I NKT cells seem to be protective, Type II NKT cells mostly suppress tumor immunity.Citation39,Citation41
In terms of cytokine production, activated NKT cells are potent providers of IFNγ and IL-4 (and, to lesser extent, of IL-17). In the B16 metastatic melanoma model, a dual role of NKT cells was linked to immune suppressive IL-4 production by the thymus-derived subpopulation; and protective IFNγ production by liver-derived Type I NKT cells.Citation42
Based on the pre-clinical evidence for an anti-tumor role of type I NKT cells, and the availability of a specific TCR agonist, α-Gal-Cer, several clinical trials have attempted to activate endogenous iNKT cells, or—more promising given by relative rarity of NKT cells in humans—perform ACT with (ex vivo expanded) Type I NKT cells. However, the clinical effects of αGal-Cer or NKT ACT have been very limited,Citation39 thus illustrating the difficulty in translating findings from animal models of cancer into improved immunotherapies.
The Inflammatory Phagocytes: TAMs and TANs
Macrophages and neutrophils are important myeloid cells of the innate immune system and major drivers of inflammatory responses. Given the long-established association between cancer and inflammation, it is not surprising that tumor-associated macrophages (TAMs) and neutrophils (TANs) can have great impact on the course of tumor progression. While most studies have associated TAM and TAN infiltration with promotion of tumor cell growth, some other reports have proposed some anti-tumor roles. Once again, these opposing behaviors may be explained by heterogeneous TAM and TAN phenotypes, with distinct intra-tumor dynamics in various models.
Mirroring Th1/ Th2 polarization of CD4+ T cells, two distinct subsets of macrophages have been recognized: the “classical” activated (M1) macrophage phenotype and the “alternatively” activated (M2) macrophage phenotype.Citation43 IFNγ drives the polarization toward M1 macrophages, which are characterized by abundant production of TNF, IL-12 and IL-23, CXCL9 and CXCL10, reactive nitrogen and oxygen species; and by high expression of MHC class II and costimulatory molecules (making them efficient antigen-presenting cells).Citation44 Conversely, IL-4 polarizes macrophages toward the M2 phenotype, which is associated with low levels of IL-12 but high levels of IL-10, IL-1RA and IL-1 decoy receptor. M2 cells also produce CCL17, CCL22 and CCL24, which results in the recruitment of Tregs and Th2 cells, eosinophils and basophils.Citation44
The balance between M1 and M2 phenotypes seems to be controlled by NFκB signaling. Thus, NFκB targeting switched macrophages from an M2 to an M1 phenotype and led to ovarian tumor regression in vivo.Citation45 Nonetheless, the most frequent TAM phenotype seems to be M2.Citation43 Consistent with this, TAM depletion was associated with improved anti-tumor immunity in models of metastatic breast, colon and non-small lung cancers.Citation46 The pro-tumor roles of M2 macrophages derive from various molecular mechanisms, including the production of the pro-angiogenic mediator semaphoring 4DCitation47 and the invasive proteases cathepsins B and S.Citation48
In the case of neutrophils, besides secreting cytokines and chemokines (such as IL-1β, IL-8, and IL-12), they produce large amounts of proteinases that remodel the extracellular matrix and promote the release of pro-angiogenic VEGF, thus supporting tumor cell growth and invasiveness.Citation49 Particularly important neutrophil proteinases are elastaseCitation50 and matrix metalloproteinases MMP-8 and MMP-9.Citation51
Despite being widely accepted as pro-tumor mediators based on multiple pre-clinical and clinical studies,Citation49 a dual nature of tumor-infiltrating neutrophils has also been suggested recently.Citation52,Citation53 Thus, anti-tumor N1 and pro-tumor N2 subsets were described and modulated within tumors by TGFβCitation52 or IFNβ.Citation54 Consistent with such a complex neutrophil activity within the tumor microenvironment, the concentration of reactive oxygen species also seems to determine either pro-tumor (genotoxicity at modest concentrations) or anti-tumor (cytotoxicity at high concentrations) effects.Citation49 Consequently, the depletion of total neutrophils can lead to either reducedCitation52 or increasedCitation55 tumor burden, further illustrating the globally paradoxical roles of tumor-infiltrating leukocytes.
Immunosuppressive Leukocytes: Treg and MDSCs
Myeloid-derived suppressor cells (MDSCs) represent a heterogeneous population of myeloid progenitors and precursors of macrophages, granulocytes and dendritic cells, which are better characterized by their strong capacity to inhibit both innate and acquired immunityCitation56 particularly T-cell responses.Citation57 Murine MDSCs can be identified by the expression of Gr1 (includes Ly6C and Ly6G, macrophage and neutrophil markers, respectively) and CD11b (characteristic of macrophages). In humans, MDSCs are characterized by a CD11b+ CD33+ CD34+ CD14- HLA-DR- phenotype. Tumors produce various factors that promote MDSC expansion, such as IL-6, VEGF or GM-CSF, whereas they get further activated by local IFNγ, IL-1β or Toll-like receptor (TLR) signals.Citation57
MDSCs use a diversity of mechanisms to suppress T-cell function, including the uptake of arginine and cysteine (essential amino acid for T cell activation) and the nitration of the TCR.Citation56 In addition, MDSCs have been recently shown to directly support tumor growth by promoting the epithelial-to-mesenchymal transition in melanocytes.Citation58
The possibility of improving anti-tumor immune responses by targeting MDSCs has been explored in pre-clinical models. One of the chemical drugs that seem to be more effective for MDSC depletion was 5-fluorouracil (5-FU). In a model of thymoma EL4 cells transplanted subcutaneously, tumor-bearing mice treated with 5-FU showed reduced number of MDSC in tumor lesions. This associated with prolonged mouse survival and enhanced intratumoral CD8+ T cell antigen-specific capacity to produce IFNγ.Citation59 Interestingly, combination therapy with an agent (cyclophosphamide, CTX) that reduces Tregs led to a synergistic protective effect. Consistent with this, another study showed that inhibition of MDSC and Treg function within B16 melanomas using blocking antibodies to CTLA-4 (already in clinical use—ipilimumab—in late-stage melanoma) and to PD-1 reduced tumor development and increased mouse survival.Citation60
Foxp3+ Tregs are well known to suppress the activation, proliferation and effector functions (such as cytokine production) of a wide range of immune cells, including αβ and γδ T cells, NK and NKT cells, B cells, macrophages and DCs. Suppressive functions displayed by Tregs include contact-dependent mechanisms, such as those that involve CTLA-4, PD-1 and GITR; and cytokine-mediated mechanisms such as TGFβ, IL-10 and IL-35.Citation61 TGFβ is particularly critical since, besides being strongly immunosuppressive, creates a potent positive feedback mechanism by instructing the differentiation of “inducible” Tregs.Citation1
Experimental Treg depletion has been usually accomplished using anti-CD25 monoclonal antibodies, since there is a good correlation between CD25 and Foxp3 expression within CD4+ T cells (although activated effector cells also upregulate CD25). Prophylactic Treg depletion in renal cell carcinoma and MCA carcinoma was shown to reduce tumor growth, with protection being dependent on CD8+ and NK cells.Citation62,Citation63
While most studies have concentrated on the immunosuppressive function of Tregs, two recent reports have shown that they can also act by directly promoting tumor growth and dissemination. Thus, Treg TILs in ovarian cancer they secrete VEGF that promotes endothelial cell proliferation;Citation64 and in breast cancer they produce RANKL, which associates with lung metastasis.Citation65 Importantly, the latter study is one of many that demonstrates that Treg accumulation within tumors is a marker for poor clinical outcome.Citation66
The Prognostic Value of Tumor-Infiltrating Leukocytes in the Clinic
Although a favorable association of high numbers of TILs in the primary tumors had been generally reported for decades in many human cancers, TILs had never reached the level of recognized prognostic marker (or proof for cancer immunosurveillance) probably due to their phenotypic and functional heterogeneity.Citation67 The recent observations that specific immune parameters have better prognostic value than standard staging systems, highlights the importance of the endogenous immune response in determining the clinical outcome. This may help to modify current classifications, and—most importantly—to identify the patients who would benefit the most from adjuvant immunotherapy.
Considering the data reviewed above, it is tempting to assume that good prognosis associates (for example) with CD8+ and NK cells, whereas bad prognosis is linked to the accumulation of Tregs and MDSCs. Moreover, given the functional heterogeneity within many leukocyte populations, clearly distinct outcomes could be expected from Th1 vs. Th2 or Th17 CD4+ subsets, M1 vs. M2 macrophages, N1 vs. N2 neutrophils. This level of refinement is obviously incompatible with traditional immunohistochemistry of cancer patient samples, thus requiring additional techniques like flow cytometry and molecular biology to provide an adequate characterization of tumor-infiltrating leukocytes. Furthermore, detailed imaging may also be important as to define the localization of TILs within the tumor mass. For example, in a pre-clinical model, CD8+ T cells were recently shown to be trapped in the stroma and thus excluded from the core of tumor due to post-translational modifications (nitration) of the chemokine CCL2.Citation68 Of note, novel drugs that inhibited CCL2 nitration facilitated CD8+ T cell infiltration and tumor regression.
The most comprehensive clinical studies correlating tumor-infiltrating leukocytes with disease outcome have been performed in colorectal cancer, where the general conclusion has been that disease free overall survival is positively associated with a coordinated Th1/ CD8+ T cell infiltrationCitation67 (). A similar result was reached for breast cancer;Citation69,Citation70 and for hepatocellular carcinoma, where NK markers and the chemokines CCL2, CCL5 and CXCL10 were additional immune signatures predictive of patient survival (at early stages of the disease).Citation71
Table 1. Tumor-infiltrating leukocytes associated with good prognosis for cancer patients
By contrast, Treg infiltration has been generally associated with poor prognosis (). In ovarian carcinoma, melanoma, breast cancer, Hodgkin lymphoma and glioblastoma, the presence and frequency of Tregs correlated with tumor grade and with reduced patient survival.Citation66 These studies also highlighted the potential role for CCL17 and CCL22 (ligands for the chemokine receptor CCR4) in recruiting Tregs into tumors. The combined value of quantifying both CD8+ and Treg TIL (antagonistic) subsets as prognostic of disease-free survival was demonstrated in hepatocellular carcinomaCitation72,Citation73 and colorectal cancer.Citation74 However, in some cancer types, such as colorectalCitation75 and head and neck carcinomas,Citation76 Treg accumulation within tumors has been associated with favorable prognosis. This was suggested to be due to a dominant effect in suppressing infection-associated inflammation at mucosal interfaces.Citation77 Nonetheless, positive associations between survival and Treg numbers were also observed upon immunohistochemical analysis of biopsies from four types of lymphoma patients.Citation78,Citation79
Table 2. Tumor-infiltrating leucocytes associated with poor prognosis for cancer patients
Whereas Th2 infiltration has been associated with poor prognosis in pancreatic cancer,Citation80 the role of Th17 TILs in human cancer is much more controversial. On one hand, Th17 cell infiltration has been correlated with poor prognosis in prostate cancerCitation81 and in hepatocellular carcinoma;Citation82 on the other, it has been associated with better overall survival in ovarian cancerCitation83 and in esophageal squamous cell carcinoma.Citation84 While the reasons for these discrepancies are unclear, it may be interesting to assess the co-production of IL-17 and IFNγ by Th17 cells, as well as their association with CD8+ T cell recruitment.
Finally, a recent study attempted to integrate the prognostic values of Th1, Th2 and Th17 TILs by hierarchical clustering of signature gene transcripts in colorectal tumor specimens. The results showed that: the Th2 cluster did not correlate with prognosis; patients with high expression of the Th1 cluster had prolonged disease-free survival; and patients with high expression of Th17 cluster had poor prognosis.Citation85 In the future, we believe that will be highly informative and important to collect similar data in many other cancer types.
Conclusions
The identification of highly diverse tumor-infiltrating leukocyte subsets and their distinct, sometimes antagonistic, functions in the tumor niche has been an important development in Oncoimmunology. This has allowed a better dissection and understanding of the interactions between immune components and tumor cells, not only in animal models but also in patients. We are now approaching an era where immune parameters will likely constitute some of the best prognostic markers for cancer progression/ regression. This will also allow a more insightful selection of patients to undergo immunotherapy as adjuvant treatment. Notwithstanding, many basic aspects of TIL biology still need to be clarified as to resolve key controversies in the field, such as the paradoxical behaviors of Th17 and γδ T cells (among others) in distinct cancer models/ types. Clinical studies must now routinely include in-depth population phenotyping and gene expression analysis in order to address the striking heterogeneity of the immune infiltrates. These future directions will be crucial to clinically promote an anti-tumor microenvironment and thus increase the success of cancer immunotherapy.
| Abbreviations: | ||
| TIL | = | tumor-infiltrating lymphocyte |
| Th | = | helper T cell |
| Treg | = | regulatory T cell |
| NK | = | natural killer cell |
| MDSC | = | myeloid-derived suppressor cell |
| IFN | = | interferon |
| IL | = | interleukin |
| VEGF | = | vascular endothelial growth factor |
| ACT | = | adoptive cell therapy |
Acknowledgments
We thank Dan Pennington and Margarida Rei for inspiring discussions on this topic. Our research is supported by the European Research Council and the European Molecular Biology Organization (Young Investigator Programme); T.L. is also supported by a PhD fellowship from Fundação para a Ciência e Tecnologia (Portugal).
References
- Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limón P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol 2010; 10:554 - 67; http://dx.doi.org/10.1038/nri2808; PMID: 20616810
- Gomes AQ, Correia DV, Silva-Santos B. Non-classical major histocompatibility complex proteins as determinants of tumour immunosurveillance. EMBO Rep 2007; 8:1024 - 30; http://dx.doi.org/10.1038/sj.embor.7401090; PMID: 17972902
- Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity 2008; 28:571 - 80; http://dx.doi.org/10.1016/j.immuni.2008.02.016; PMID: 18394936
- Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, et al. Innate or adaptive immunity? The example of natural killer cells. Science 2011; 331:44 - 9; http://dx.doi.org/10.1126/science.1198687; PMID: 21212348
- Fregni G, Perier A, Avril MF, Caignard A. NK cells sense tumors, course of disease and treatments. OncoImmunology 2012; 1:38 - 47; http://dx.doi.org/10.4161/onci.1.1.18312
- Ljunggren HG, Malmberg KJ. Prospects for the use of NK cells in immunotherapy of human cancer. Nat Rev Immunol 2007; 7:329 - 39; http://dx.doi.org/10.1038/nri2073; PMID: 17438573
- Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 2011; 71:5412 - 22; http://dx.doi.org/10.1158/0008-5472.CAN-10-4179; PMID: 21708957
- Delahaye NF, Rusakiewicz S, Martins I, Ménard C, Roux S, Lyonnet L, et al. Alternatively spliced NKp30 isoforms affect the prognosis of gastrointestinal stromal tumors. Nat Med 2011; 17:700 - 7; http://dx.doi.org/10.1038/nm.2366; PMID: 21552268
- Klebanoff CA, Acquavella N, Yu Z, Restifo NP. Therapeutic cancer vaccines: are we there yet?. Immunol Rev 2011; 239:27 - 44; http://dx.doi.org/10.1111/j.1600-065X.2010.00979.x; PMID: 21198663
- Rosenberg SA. Cell transfer immunotherapy for metastatic solid cancer--what clinicians need to know. Nat Rev Clin Oncol 2011; 8:577 - 85; http://dx.doi.org/10.1038/nrclinonc.2011.116; PMID: 21808266
- Thompson ED, Enriquez HL, Fu YX, Engelhard VH. Tumor masses support naive T cell infiltration, activation, and differentiation into effectors. J Exp Med 2010; 207:1791 - 804; http://dx.doi.org/10.1084/jem.20092454; PMID: 20660615
- Hinrichs CS, Borman ZA, Cassard L, Gattinoni L, Spolski R, Yu Z, et al. Adoptively transferred effector cells derived from naive rather than central memory CD8+ T cells mediate superior antitumor immunity. Proc Natl Acad Sci U S A 2009; 106:17469 - 74; http://dx.doi.org/10.1073/pnas.0907448106; PMID: 19805141
- Norell H, Zhang Y, McCracken J, Martins da Palma T, Lesher A, Liu Y, et al. CD34-based enrichment of genetically engineered human T cells for clinical use results in dramatically enhanced tumor targeting. Cancer Immunol Immunother 2010; 59:851 - 62; http://dx.doi.org/10.1007/s00262-009-0810-8; PMID: 20052466
- Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011; 365:725 - 33; http://dx.doi.org/10.1056/NEJMoa1103849; PMID: 21830940
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2011; http://dx.doi.org/10.1182/blood-2011-10-384388; PMID: 22160384
- Knutson KL, Disis ML. Tumor antigen-specific T helper cells in cancer immunity and immunotherapy. Cancer Immunol Immunother 2005; 54:721 - 8; http://dx.doi.org/10.1007/s00262-004-0653-2; PMID: 16010587
- Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, et al. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res 2010; 70:2697 - 706; http://dx.doi.org/10.1158/0008-5472.CAN-09-2982; PMID: 20215523
- Martin-Orozco N, Muranski P, Chung Y, Yang XO, Yamazaki T, Lu S, et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 2009; 31:787 - 98; http://dx.doi.org/10.1016/j.immuni.2009.09.014; PMID: 19879162
- Kryczek I, Wei S, Szeliga W, Vatan L, Zou W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009; 114:357 - 9; http://dx.doi.org/10.1182/blood-2008-09-177360; PMID: 19289853
- Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008; 112:362 - 73; http://dx.doi.org/10.1182/blood-2007-11-120998; PMID: 18354038
- Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, et al. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity 2011; 35:972 - 85; http://dx.doi.org/10.1016/j.immuni.2011.09.019; PMID: 22177921
- Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med 2009; 206:1457 - 64; http://dx.doi.org/10.1084/jem.20090207; PMID: 19564351
- Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res 2010; 70:10112 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-10-0775; PMID: 21159633
- Chae WJ, Gibson TF, Zelterman D, Hao L, Henegariu O, Bothwell AL. Ablation of IL-17A abrogates progression of spontaneous intestinal tumorigenesis. Proc Natl Acad Sci U S A 2010; 107:5540 - 4; http://dx.doi.org/10.1073/pnas.0912675107; PMID: 20212110
- Silva-Santos B. Promoting angiogenesis within the tumor microenvironment: the secret life of murine lymphoid IL-17-producing gammadelta T cells. Eur J Immunol 2010; 40:1873 - 6; http://dx.doi.org/10.1002/eji.201040707; PMID: 20549671
- Lochner M, Peduto L, Cherrier M, Sawa S, Langa F, Varona R, et al. In vivo equilibrium of proinflammatory IL-17+ and regulatory IL-10+ Foxp3+ RORgamma t+ T cells. J Exp Med 2008; 205:1381 - 93; http://dx.doi.org/10.1084/jem.20080034; PMID: 18504307
- Wakita D, Sumida K, Iwakura Y, Nishikawa H, Ohkuri T, Chamoto K, et al. Tumor-infiltrating IL-17-producing gammadelta T cells support the progression of tumor by promoting angiogenesis. Eur J Immunol 2010; 40:1927 - 37; http://dx.doi.org/10.1002/eji.200940157; PMID: 20397212
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing gamma delta T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491 - 503; http://dx.doi.org/10.1084/jem.20100269; PMID: 21383056
- Mattarollo SR, Loi S, Duret H, Ma Y, Zitvogel L, Smyth MJ. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res 2011; 71:4809 - 20; http://dx.doi.org/10.1158/0008-5472.CAN-11-0753; PMID: 21646474
- Girardi M, Oppenheim DE, Steele CR, Lewis JM, Glusac E, Filler R, et al. Regulation of cutaneous malignancy by gammadelta T cells. Science 2001; 294:605 - 9; http://dx.doi.org/10.1126/science.1063916; PMID: 11567106
- Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med 2003; 198:433 - 42; http://dx.doi.org/10.1084/jem.20030584; PMID: 12900519
- Street SE, Hayakawa Y, Zhan Y, Lew AM, MacGregor D, Jamieson AM, et al. Innate immune surveillance of spontaneous B cell lymphomas by natural killer cells and gammadelta T cells. J Exp Med 2004; 199:879 - 84; http://dx.doi.org/10.1084/jem.20031981; PMID: 15007091
- Liu Z, Eltoum IE, Guo B, Beck BH, Cloud GA, Lopez RD. Protective immunosurveillance and therapeutic antitumor activity of gammadelta T cells demonstrated in a mouse model of prostate cancer. J Immunol 2008; 180:6044 - 53; PMID: 18424725
- Ribot JC, deBarros A, Pang DJ, Neves JF, Peperzak V, Roberts SJ, et al. CD27 is a thymic determinant of the balance between interferon-gamma- and interleukin 17-producing gammadelta T cell subsets. Nat Immunol 2009; 10:427 - 36; http://dx.doi.org/10.1038/ni.1717; PMID: 19270712
- Gomes AQ, Martins DS, Silva-Santos B. Targeting γδ T lymphocytes for cancer immunotherapy: from novel mechanistic insight to clinical application. Cancer Res 2010; 70:10024 - 7; http://dx.doi.org/10.1158/0008-5472.CAN-10-3236; PMID: 21159627
- Lança T, Correia DV, Moita CF, Raquel H, Neves-Costa A, Ferreira C, et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood 2010; 115:2407 - 11; http://dx.doi.org/10.1182/blood-2009-08-237123; PMID: 20101024
- Correia DV, Fogli M, Hudspeth K, da Silva MG, Mavilio D, Silva-Santos B. Differentiation of human peripheral blood Vδ1+ T cells expressing the natural cytotoxicity receptor NKp30 for recognition of lymphoid leukemia cells. Blood 2011; 118:992 - 1001; http://dx.doi.org/10.1182/blood-2011-02-339135; PMID: 21633088
- D’Cruz LM, Yang CY, Goldrath AW. Transcriptional regulation of NKT cell development and homeostasis. Curr Opin Immunol 2010; 22:199 - 205; http://dx.doi.org/10.1016/j.coi.2010.01.014; PMID: 20171073
- Exley MA, Nakayama T. NKT-cell-based immunotherapies in clinical trials. Clin Immunol 2011; 140:117 - 8; http://dx.doi.org/10.1016/j.clim.2011.04.015; PMID: 21592864
- Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood 2009; 113:6382 - 5; http://dx.doi.org/10.1182/blood-2009-01-198564; PMID: 19234138
- Terabe M, Swann J, Ambrosino E, Sinha P, Takaku S, Hayakawa Y, et al. A nonclassical non-Valpha14Jalpha18 CD1d-restricted (type II) NKT cell is sufficient for down-regulation of tumor immunosurveillance. J Exp Med 2005; 202:1627 - 33; http://dx.doi.org/10.1084/jem.20051381; PMID: 16365146
- Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 2005; 202:1279 - 88; http://dx.doi.org/10.1084/jem.20050953; PMID: 16275765
- Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011; 41:2522 - 5; http://dx.doi.org/10.1002/eji.201141894; PMID: 21952810
- Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 2010; 11:889 - 96; http://dx.doi.org/10.1038/ni.1937; PMID: 20856220
- Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, et al. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med 2008; 205:1261 - 8; http://dx.doi.org/10.1084/jem.20080108; PMID: 18490490
- Luo Y, Zhou H, Krueger J, Kaplan C, Lee SH, Dolman C, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J Clin Invest 2006; 116:2132 - 41; http://dx.doi.org/10.1172/JCI27648; PMID: 16862213
- Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, et al. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med 2008; 205:1673 - 85; http://dx.doi.org/10.1084/jem.20072602; PMID: 18559453
- Gocheva V, Wang HW, Gadea BB, Shree T, Hunter KE, Garfall AL, et al. IL-4 induces cathepsin protease activity in tumor-associated macrophages to promote cancer growth and invasion. Genes Dev 2010; 24:241 - 55; http://dx.doi.org/10.1101/gad.1874010; PMID: 20080943
- Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 2010; 9:1732 - 7; http://dx.doi.org/10.4161/cc.9.9.11297; PMID: 20404546
- Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE, et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010; 16:219 - 23; http://dx.doi.org/10.1038/nm.2084; PMID: 20081861
- Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell 2010; 141:52 - 67; http://dx.doi.org/10.1016/j.cell.2010.03.015; PMID: 20371345
- Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009; 16:183 - 94; http://dx.doi.org/10.1016/j.ccr.2009.06.017; PMID: 19732719
- Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2011; http://dx.doi.org/10.1016/j.critrevonc.2011.06.004; PMID: 21798756
- Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest 2010; 120:1151 - 64; http://dx.doi.org/10.1172/JCI37223; PMID: 20237412
- Granot Z, Henke E, Comen EA, King TA, Norton L, Benezra R. Tumor entrained neutrophils inhibit seeding in the premetastatic lung. Cancer Cell 2011; 20:300 - 14; http://dx.doi.org/10.1016/j.ccr.2011.08.012; PMID: 21907922
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499 - 506; http://dx.doi.org/10.4049/jimmunol.0802740; PMID: 19342621
- Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol 2011; 32:19 - 25; http://dx.doi.org/10.1016/j.it.2010.10.002; PMID: 21067974
- Toh B, Wang X, Keeble J, Sim WJ, Khoo K, Wong WC, et al. Mesenchymal transition and dissemination of cancer cells is driven by myeloid-derived suppressor cells infiltrating the primary tumor. PLoS Biol 2011; 9:e1001162; http://dx.doi.org/10.1371/journal.pbio.1001162; PMID: 21980263
- Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res 2010; 70:3052 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-09-3690; PMID: 20388795
- Curran MA, Montalvo W, Yagita H, Allison JP. PD-1 and CTLA-4 combination blockade expands infiltrating T cells and reduces regulatory T and myeloid cells within B16 melanoma tumors. Proc Natl Acad Sci U S A 2010; 107:4275 - 80; http://dx.doi.org/10.1073/pnas.0915174107; PMID: 20160101
- Sakaguchi S, Miyara M, Costantino CM, Hafler DA. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 2010; 10:490 - 500; http://dx.doi.org/10.1038/nri2785; PMID: 20559327
- Teng MW, Swann JB, von Scheidt B, Sharkey J, Zerafa N, McLaughlin N, et al. Multiple antitumor mechanisms downstream of prophylactic regulatory T-cell depletion. Cancer Res 2010; 70:2665 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-09-1574; PMID: 20332236
- Jacobs JF, Nierkens S, Figdor CG, de Vries IJ, Adema GJ. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy?. Lancet Oncol 2012; 13:e32 - 42; http://dx.doi.org/10.1016/S1470-2045(11)70155-3; PMID: 22225723
- Facciabene A, Peng X, Hagemann IS, Balint K, Barchetti A, Wang LP, et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and T(reg) cells. Nature 2011; 475:226 - 30; http://dx.doi.org/10.1038/nature10169; PMID: 21753853
- Tan W, Zhang W, Strasner A, Grivennikov S, Cheng JQ, Hoffman RM, et al. Tumour-infiltrating regulatory T cells stimulate mammary cancer metastasis through RANKL-RANK signalling. Nature 2011; 470:548 - 53; http://dx.doi.org/10.1038/nature09707; PMID: 21326202
- Quezada SA, Peggs KS, Simpson TR, Allison JP. Shifting the equilibrium in cancer immunoediting: from tumor tolerance to eradication. Immunol Rev 2011; 241:104 - 18; http://dx.doi.org/10.1111/j.1600-065X.2011.01007.x; PMID: 21488893
- Fridman WH, Mlecnik B, Bindea G, Pagès F, Galon J. Immunosurveillance in human non-viral cancers. Curr Opin Immunol 2011; 23:272 - 8; http://dx.doi.org/10.1016/j.coi.2010.12.011; PMID: 21237631
- Molon B, Ugel S, Del Pozzo F, Soldani C, Zilio S, Avella D, et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J Exp Med 2011; 208:1949 - 62; http://dx.doi.org/10.1084/jem.20101956; PMID: 21930770
- Martinet L, Garrido I, Filleron T, Le Guellec S, Bellard E, Fournie JJ, et al. Human solid tumors contain high endothelial venules: association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res 2011; 71:5678 - 87; http://dx.doi.org/10.1158/0008-5472.CAN-11-0431; PMID: 21846823
- Ladoire S, Arnould L, Mignot G, Apetoh L, Rébé C, Martin F, et al. T-bet expression in intratumoral lymphoid structures after neoadjuvant trastuzumab plus docetaxel for HER2-overexpressing breast carcinoma predicts survival. Br J Cancer 2011; 105:366 - 71; http://dx.doi.org/10.1038/bjc.2011.261; PMID: 21750556
- Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, et al. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut 2012; 61:427 - 38; http://dx.doi.org/10.1136/gutjnl-2011-300509; PMID: 21930732
- Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS, et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007; 25:2586 - 93; http://dx.doi.org/10.1200/JCO.2006.09.4565; PMID: 17577038
- Chen KJ, Zhou L, Xie HY, Ahmed TE, Feng XW, Zheng SS. Intratumoral regulatory T cells alone or in combination with cytotoxic T cells predict prognosis of hepatocellular carcinoma after resection. Med Oncol 2011; http://dx.doi.org/10.1007/s12032-011-0006-x; PMID: 21678026
- Suzuki H, Chikazawa N, Tasaka T, Wada J, Yamasaki A, Kitaura Y, et al. Intratumoral CD8(+) T/FOXP3 (+) cell ratio is a predictive marker for survival in patients with colorectal cancer. Cancer Immunol Immunother 2010; 59:653 - 61; http://dx.doi.org/10.1007/s00262-009-0781-9; PMID: 19908042
- Correale P, Rotundo MS, Del Vecchio MT, Remondo C, Migali C, Ginanneschi C, et al. Regulatory (FoxP3+) T-cell tumor infiltration is a favorable prognostic factor in advanced colon cancer patients undergoing chemo or chemoimmunotherapy. J Immunother 2010; 33:435 - 41; http://dx.doi.org/10.1097/CJI.0b013e3181d32f01; PMID: 20386463
- Badoual C, Hans S, Rodriguez J, Peyrard S, Klein C, Agueznay NelH, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res 2006; 12:465 - 72; http://dx.doi.org/10.1158/1078-0432.CCR-05-1886; PMID: 16428488
- Ladoire S, Martin F, Ghiringhelli F. Prognostic role of FOXP3+ regulatory T cells infiltrating human carcinomas: the paradox of colorectal cancer. Cancer Immunol Immunother 2011; 60:909 - 18; http://dx.doi.org/10.1007/s00262-011-1046-y; PMID: 21644034
- Tzankov A, Meier C, Hirschmann P, Went P, Pileri SA, Dirnhofer S. Correlation of high numbers of intratumoral FOXP3+ regulatory T cells with improved survival in germinal center-like diffuse large B-cell lymphoma, follicular lymphoma and classical Hodgkin’s lymphoma. Haematologica 2008; 93:193 - 200; http://dx.doi.org/10.3324/haematol.11702; PMID: 18223287
- Kim WY, Jeon YK, Kim TM, Kim JE, Kim YA, Lee SH, et al. Increased quantity of tumor-infiltrating FOXP3-positive regulatory T cells is an independent predictor for improved clinical outcome in extranodal NK/T-cell lymphoma. Ann Oncol 2009; 20:1688 - 96; http://dx.doi.org/10.1093/annonc/mdp056; PMID: 19542249
- De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med 2011; 208:469 - 78; http://dx.doi.org/10.1084/jem.20101876; PMID: 21339327
- Sfanos KS, Bruno TC, Maris CH, Xu L, Thoburn CJ, DeMarzo AM, et al. Phenotypic analysis of prostate-infiltrating lymphocytes reveals TH17 and Treg skewing. Clin Cancer Res 2008; 14:3254 - 61; http://dx.doi.org/10.1158/1078-0432.CCR-07-5164; PMID: 18519750
- Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L, et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009; 50:980 - 9; http://dx.doi.org/10.1016/j.jhep.2008.12.033; PMID: 19329213
- Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 2009; 114:1141 - 9; http://dx.doi.org/10.1182/blood-2009-03-208249; PMID: 19470694
- Lv L, Pan K, Li XD, She KL, Zhao JJ, Wang W, et al. The accumulation and prognosis value of tumor infiltrating IL-17 producing cells in esophageal squamous cell carcinoma. PLoS One 2011; 6:e18219; http://dx.doi.org/10.1371/journal.pone.0018219; PMID: 21483813
- Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res 2011; 71:1263 - 71; http://dx.doi.org/10.1158/0008-5472.CAN-10-2907; PMID: 21303976
- Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol 2009; 27:5944 - 51; http://dx.doi.org/10.1200/JCO.2008.19.6147; PMID: 19858404
- Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, et al. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 2011; 29:610 - 8; http://dx.doi.org/10.1200/JCO.2010.30.5425; PMID: 21245428
- Cho Y, Miyamoto M, Kato K, Fukunaga A, Shichinohe T, Kawarada Y, et al. CD4+ and CD8+ T cells cooperate to improve prognosis of patients with esophageal squamous cell carcinoma. Cancer Res 2003; 63:1555 - 9; PMID: 12670904
- Schumacher K, Haensch W, Röefzaad C, Schlag PM. Prognostic significance of activated CD8(+) T cell infiltrations within esophageal carcinomas. Cancer Res 2001; 61:3932 - 6; PMID: 11358808
- Raspollini MR, Castiglione F, Rossi Degl’innocenti D, Amunni G, Villanucci A, Garbini F, et al. Tumour-infiltrating gamma/delta T-lymphocytes are correlated with a brief disease-free interval in advanced ovarian serous carcinoma. Ann Oncol 2005; 16:590 - 6; http://dx.doi.org/10.1093/annonc/mdi112; PMID: 15699022
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004; 10:942 - 9; http://dx.doi.org/10.1038/nm1093; PMID: 15322536
- Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, et al. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol 2006; 24:5373 - 80; http://dx.doi.org/10.1200/JCO.2006.05.9584; PMID: 17135638
- Gabitass RF, Annels NE, Stocken DD, Pandha HA, Middleton GW. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol Immunother 2011; 60:1419 - 30; http://dx.doi.org/10.1007/s00262-011-1028-0; PMID: 21644036
- Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol 2012; 65:159 - 63; http://dx.doi.org/10.1136/jclinpath-2011-200355; PMID: 22049225
- Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, von der Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol 2009; 27:4709 - 17; http://dx.doi.org/10.1200/JCO.2008.18.9498; PMID: 19720929