Abstract
Vav1 is expressed exclusively in hematopoietic cells and is required for T cell development and activation. Vav1-deficient mice show thymic hypocellularity due to a partial block during thymocyte development at the DN3 stage and between the double positive (DP) and single positive (SP) transition. Vav1 has been shown to play a significant role in several non-hematopoietic tumors but its role in leukemogenesis is unknown. To address this question, we investigated the role of Vav1 in retrovirus-induced T cell leukemogenesis. Infection of Vav1-deficient mice with the Moloney strain of murine leukemia virus (M-MuLV) significantly affected tumor phenotype without modulating tumor incidence or latency. M-MuLV-infected Vav1-deficient mice showed reduced splenomegaly, higher hematocrit levels and hypertrophic thymi. Notably, Vav1-deficient mice with M-MuLV leukemias presented with markedly lower TCRβ/CD3 levels, indicating that transformation occurred at an earlier stage of T cell development than in WT mice. Thus, impaired T cell development modulates the outcome of retrovirus-induced T cell leukemias, demonstrating a link between T cell development and T cell leukemogenesis.
Keywords: :
Introduction
Vav1, the first member of the Vav family to be described, is expressed exclusively in hematopoietic and trophoblast cells. Vav-family proteins share a tandem arrangement of a Dbl-homology (DH) domain followed by a pleckstrin homology (PH) domain. The DH domains of these proteins possess guanine nucleotide exchange factor (GEF) activity, which mediates GDP exchange for GTP on Rho-family GTPases, thereby causing activation of these GTPases. Some Rho proteins are overexpressed and hyperactivated in a number of human tumors.Citation1 As gain-of-function mutations in Rho family GTP binding proteins, such as those found in ras, are very rare in human cancers, inhibition of GTPase-activating proteins (GAPs) or expression of GEFs may account for the hyperactivation of Rho family signaling in cancer.Citation2 In fact, Vav1, similarly to other GEFs, was originally isolated as an oncogeneCitation3 and has been shown to play a role in tumorigenesis of different origins.Citation4 Vav1 is aberrantly expressed in 53% of primary pancreatic adenocarcinomas due to demethylation of the gene promoter, and is associated with decreased patient survival.Citation5 Moreover, it contributes to the tumorigenic properties of neuroblastoma,Citation6 melanomaCitation7 and pancreatic cancer cells.Citation5 In the latter, the role of Vav1 is mediated by its GEF activity with a subsequent activation of Rac1, PAK1, NF-κB and an upregulation of cyclin D1. Azathioprine, used in the treatment of lymphocytic leukemias, is an inhibitor of nucleotide biosynthesis but also blocks Vav1-induced Rac activation.Citation8,Citation9
Vav1−/− mice are viable and fertile,Citation10 but they present with an abnormal hematopoiesis: the overall size of the thymus is reduced by 50% due to a reduction in the number of double positive (DP) thymocytes, albeit with normal numbers of double negative (DN) thymocytes. Analyses of DN thymocytes have demonstrated that there is a block between the DN3 (CD44-CD25+) and DN4 (CD44-CD25-) stages, at the β-selection checkpoint. This observation strongly suggests that in the absence of Vav1, pre-TCR signaling is impaired. Other steps though are impaired as well since single positive (SP) cells are reduced by approximately 10-fold in Vav1−/− mice and this block between DP to SP cells is exacerbated in the absence of other Vav family proteins, Vav2 and Vav3.Citation10,Citation11 Furthermore, peripheral T cells derived from Vav1-deficient mice show a strong defect in cytoskeleton rearrangement and lipid raft organization, leading to impaired function.Citation12-Citation15
Vav1−/−, but not Vav2−/− or Vav3−/−, mice are prone to developing CD3+ lymphoblastic lymphoma-like tumors with increasing age, with 30% of mice dying as a consequence of this disease before 1 y.Citation16 Retroviruses of the murine leukemia virus (MuLV) family promote leukemogenesis mainly by insertional activation of proto-oncogene expression.Citation17 Viral enhancers act on proximal as well as distal promoters with the latter mediated via interactions facilitated by chromatin looping (reviewed in.Citation18 Thus, integrated proviruses can affect many genesCitation19 and induce leukemia in a multistep/multigene fashion, rather than through a unique mutation in a specific gene.
Because of the important role of Vav1 in tumorigenesis and T cell development, we infected newborn mice with the Moloney strain of MLV (M-MuLV), which commonly induces leukemia/lymphoma of T cell origin.Citation17,Citation20-Citation24 In neonatal mice, M-MuLV-induced leukemogenesis is particularly efficient, in part due to the absence of an antiviral immune response through tolerance-inducing mechanisms.Citation17 As the potential role of an antiviral immune response is limited in this model, we used neonatal WT and Vav1−/− mice to assess the role of Vav1 in the development of retrovirus-induced leukemias. While the absence of Vav1 did not affect disease incidence or latency, the phenotype of leukemic cells in the thymus and periphery were distinct.
Results
Development of leukemias in WT and Vav1−/− mice following M-MuLV inoculation
To investigate the role of Vav1 in T cell leukemogenesis, we inoculated newborn Vav1+/+, Vav1+/− and Vav1−/− mice intraperitoneally with M-MuLV, a retrovirus known to induce the development of T lymphomas.Citation17,Citation20-Citation23 There was no significant difference in M-MuLV-induced disease incidence or latency with approximately 50% of mice developing clinical signs of disease by day 115 post-infection (). By day 200 post-infection, all mice had developed leukemia.
Figure 1. Survival and tumor incidence in mice after M-MuLV infection. Vav1+/+ (n = 44), Vav1+/− (n = 72) and Vav1−/− (n = 48) mice were infected with M-MuLV. The graph shows the cumulative incidence of leukemia based on enlarged lymphoid organs and hematocrit levels.
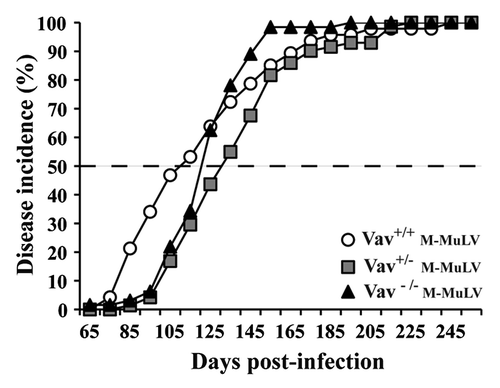
M-MuLV-induced leukemia is characterized by increases in spleen size and, to a lesser extent, the thymus.Citation17,Citation25 Despite similar survival rates, Vav1−/− mice presented with clinical characteristics that distinguished them from Vav1+/+ and Vav1+/− mice (). The hematocrits of healthy mice of all genotypes ranged between 45 to 50% (data not shown). M-MuLV-inoculated Vav1+/+ and Vav1+/− mice presented with significantly lower hematocrits, ranging from 40 to 42% (). Notably, the hematocrits in M-MuLV-inoculated Vav1−/− mice with leukemias were significantly higher (mean of 47%, p < 0.001), suggesting that erythropoiesis was less affected in these mice than in their WT counterpart or that they showed a lower percentage of leukemic T cells in the periphery.
Figure 2. Phenotype of M-MuLV-infected mice. Mice described in were analyzed for tumor phenotype. A) Hematocrits in the different mice populations were monitored one day before euthanasia. B) Spleen and thymus cellularity in M-MuLV-infected mice. C) Spleen weights in different mice populations. D) Size of thymi in different mice populations. Data were evaluated using Student’s t-test: * p < 0.05; **p < 0.01; ***p < 0.001.
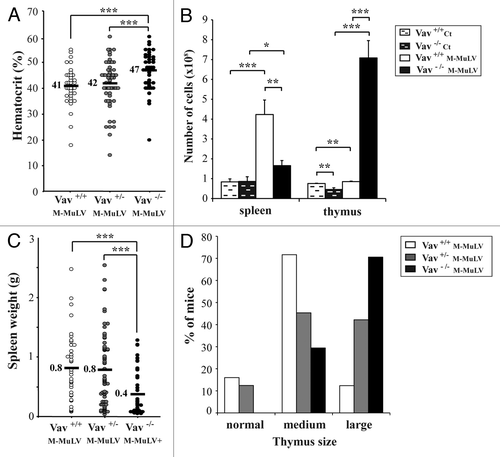
Splenic cellularity in healthy mice of all genotypes was equivalent with a weight of approximately 0.08 g (). Upon M-MuLV-induced leukemogenesis, Vav1+/+ and Vav1+/− mice developed severe splenomegaly (10-fold weight increase) that correlated with increased cellularity (). While splenomegaly was also detected in M-MuLV-infected Vav1−/− mice, their spleens were significantly smaller and showed decreased cellularity as compared with leukemic Vav1+/+ and Vav1+/− mice ( and ).
Under conditions of M-MuLV-induced leukemia, Vav1+/+ and Vav1+/− mice showed small increases in thymic size (). However, in Vav1−/− mice, which under normal conditions have reduced thymic cellularity due to a lower number of DP cells,Citation11 M-MuLV-induced leukemia resulted in a massive increase in the size of the thymus (). The thymi of Vav1−/− infected mice often invaded the entire thoracic cavity and indeed, these mice often suffered from respiratory problems during the latter stages of disease.
Distinct thymocyte profiles in Vav1+/− and Vav1−/− mice with M-MuLV-induced leukemia
M-MuLV-induced T cell leukemogenesis is often associated with the expansion and accumulation of thymocytes with an immature phenotype.Citation17,Citation25 To study the phenotype of thymocytes accumulating in the different mice cohorts, we first assessed the CD4/CD8 profiles. While profiles of thymocytes from infected mice showed some variability, they differed significantly from those of healthy mice. CD4/CD8 staining from individual mice is presented in . The majority of lymphomagenic thymi from leukemic Vav1+/+ mice harbored a high percentage of CD4-expressing cells, as also reported following infection with the radiation leukemia virus (RadLV;Citation26). In healthy Vav1−/− mice, the percentages of DN and SP thymocytes were modulated, with significantly higher levels of the former (p < 0.05) and lower levels of the latter (p < 0.05; and referenceCitation11). Leukemia development in Vav1−/− mice was associated with higher variability; approximately half the mice harbored > 40% single positive CD4 cells (SP4) while the remainder harbored < 20% SP4 cells ( and ). To study the differentiation state of these SP4 cells, we monitored TCRβ expression. In agreement with previously published data,Citation27 thymi of healthy Vav1−/− mice had a significantly lower level of TCRβ-hi cells ( and ), indicative of an immature stage of thymopoiesis as compared with WT mice where the majority of cells were mature TCRβ-hi SP4 thymocytes (49% and 73%, respectively). While TCRβ expression remained elevated on SP4 thymocytes in leukemic Vav1+/+ mice, it was undetectable on the vast majority of SP4 thymocytes from Vav1−/− lymphomagenic thymi. Altogether, our results indicate that lymphomagenic thymocytes from Vav1−/− mice have a more immature phenotype than those present in the WT counterpart.
Figure 3. Thymocyte populations in M-MuLV infected mice. A) Flow cytometry analysis of thymi from control and M-MuLV infected mice. Cells were stained with anti-CD4 and anti-CD8 antibodies to distinguish CD4-/CD8- (DN), CD4+/CD8+ (DP), CD4+/CD8- (CD4+) and CD4-/CD8+ (CD8+) populations. B) Percentages of DN, DP, CD4+ and CD8+ from representative populations of mice (Vav1+/+ control (n = 6); Vav1−/− control (n = 6); Vav1+/+ M-MuLV (n = 4); Vav1−/− M-MuLV (n = 9)). The numbers in brackets reflect the mice whose plots are depicted in panel A. C) Representative histograms showing TCRβ expression in the CD4+/CD8- subset of different mice populations. Data were evaluated using Student’s t-test: * p < 0.05; **p < 0.01; ***p < 0.001.
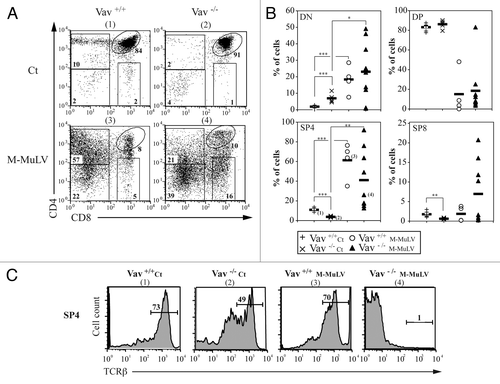
Table 1. Low surface TCRβ expression on CD4+ thymocytes in Vav1−/− mice with M-MuLV-induced leukemias. Thymocytes from control or M-MuLV infected mice were stained as described in . Within the SP4 gate (CD4+CD8-), TCRβ expression was assessed. The mean percentages ± SEM of SP4 cells expressing TCRβ are presented. Data were evaluated using Student’s t-test and TCRβ expression within the SP4 thymocyte subset in infected Vav1−/− mice was significantly lower than that detected in uninfected WT mice, uninfected Vav1−/− mice and WT infected mice. p < 0.05 compare with non infected WT mice (1), compare with WT mice (2) and compare with non infected Vav1−/− mice
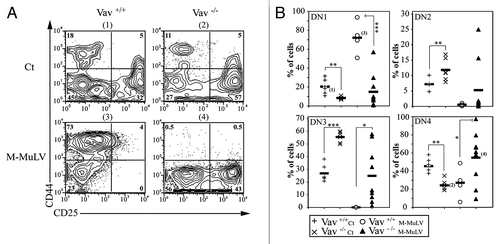
Furthermore, there was a significant increase in DN thmocytes in lymphomagenic thymi from both WT and Vav1−/− mice (). As Vav1−/− mice have a partial block between DN3 and DN4 thymocyte stages ( and ref. Citation11), it was of interest to determine whether leukemogenesis would result in the accumulation of distinct DN populations in WT and Vav1−/− mice. The majority of DN cells in leukemic WT mice had a CD44+CD25- (DN1) phenotype, while the DN profile in leukemic Vav1−/− mice revealed significantly increased percentages of CD44-CD25+ (DN3) and CD44-CD25- (DN4) cells. Thus, early stages of thymopoieis are disrupted by M-MuLV-induced leukemia, in both WT and Vav1−/− mice, but the absence of Vav1 significantly affects the development of lymphomagenic thymi.
Figure 4. Impaired thymocyte development in Vav1−/− M-MuLV mice. A) Flow cytometric analysis of thymi from control and M-MuLV infected mice. Cells were stained with CD44 and CD25 antibodies to distinguish DN1 (CD44+/CD25-), DN2 (CD44+/CD25+), DN3 (CD44-/CD25+) and DN4 (CD44-/CD25-) subsets within the CD4-CD8- population. B) Percentages of DN1, DN2, DN3 and DN4 from representative populations of mice (Vav1+/+ control (n = 6); Vav1−/− control (n = 6); Vav1+/+ M-MuLV (n = 4); Vav1−/− M-MuLV (n = 9)). The numbers in brackets reflect the mice whose plots are depicted in panel A. Data were evaluated using Student’s t-test: * p < 0.05; **p < 0.01; ***p < 0.001.
Age- and leukemia-mediated changes in the cell cycle entry of Vav1+/+ and Vav1−/− thymocytes
While the differentiation state of WT and Vav1−/− thymocytes in adult mice have long been known to be distinct,Citation10 the partition of thymocytes of different developmental stages during the newborn period is not known. This is of importance in the context of the present studies because the mice were infected with M-MuLV at 3 d of age. Furthermore, given that M-MuLV infection targets proliferating cells, it was of interest to assess the cell cycle entry of different thymocyte populations. The percentage of DP thymocytes slightly decreased in WT mice between 3 d of age (the time point of M-MuLV inoculation) and 2 mo of age () whereas it was not statistically different in Vav1−/− mice. While the presence of SP4 and SP8 thymocytes augmented with age, they remained significantly lower in Vav1−/− than WT mice.
Figure 5. Cell cycle entry of different thymocyte populations. Thymocytes were stained with the corresponding antibodies to identify CD4-/CD8- (DN), CD4+/CD8+ (DP), CD4+/CD8- (SP4) and CD4-/CD8+ (SP8) cells and with an anti-Ki67 antibody to analyze cell cycle entry. A) The total percentages of cells and the percentages of Ki67+ cells in the different populations are shown in 3-d old (white bars) and adult (black bars) mice of both genotypes . B) Comparative analysis of Ki67 expression in the different populations between Vav1−/− and wild-type mice. Data represent the mean percentages ± SEM of three independent experiments with n = 3 mice per group. Data were evaluated using Student’s t-test: * p < 0.05; **p < 0.01; ***p < 0.001.
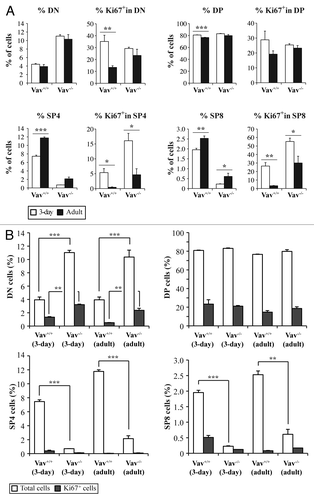
The entry of thymocytes into cell cycle, was monitored as a function of Ki67 expression, a protein upregulated during mid G1 phase. The percentage of Ki67+ cells within the DP thymocyte subset was not modulated by either age or the presence of Vav1 (). The percentages of Ki67+ DN cells decreased with age but this phenomenon was observed in both WT and Vav1−/− mice. Notably, within the SP8 population, the relative proportion of Ki67+ cells was higher during the newborn period, with greater than 50% of this subset expressing Ki67 in neonatal Vav1−/− mice. However, the total numbers of Ki67+ SP8 thymocytes were still lower in Vav1−/− mice due to the relative paucity of this population. Almost identical data were obtained in the SP4 population. Importantly, the increased percentages of Ki67+ cells within the DN and immature SP4 and SP8 thymocyte populations of Vav1−/− mice were associated with the less mature phenotype of leukemic cells in M-MLV-induced mice as compared with their wild-type counterpart ( and ).
Newborn Vav1−/− mice showed a higher percentage of Ki67+ DN cells as compared with WT mice, a feature that was also observed in adult mice ( and ref. Citation27). However, the majority of Ki67+ cells were found in the DP subpopulation, which is the largest population present in both genotypes. Interestingly though, we did not observe any increase in DP cells in lymphomagenic thymi of adult mice (), suggesting that the virus did not target this population, or, alternatively, that infected cells underwent differentiation during the transformation process. SP4 cells, which represented the most abundant population in lymphomagenic thymi from both genotypes, were only rarely Ki67+ (). Given that MuLV infection requires mitosis, these data strongly suggest it is not the SP4 population itself that was targeted by the virus but a cell at an earlier stage of differentiation.
Phenotype of peripheral leukemic cells in Vav1+/− and Vav1−/− mice
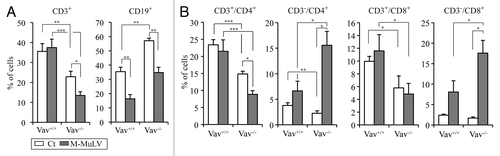
M-MuLV-induced leukemias are first produced in the thymus but there is also a peripheral involvement during later stages of the disease.Citation17,Citation21-Citation23 In order to characterize the phenotype of lymphomagenic cells in the periphery, we studied the expression of several cell markers in splenocytes from M-MuLV-inoculated mice. In WT as well as Vav1−/− leukemic mice, there was an expected decrease in the percentage of CD19+ B cells due to the presence of leukemic cells. As Vav1−/− mice have decreased percentages of peripheral T cells under normal conditions,Citation10 the relative percentage of CD19+ cells filling this nicheCitation28,Citation29 was initially increased. Somewhat surprisingly though, there was a decreased percentage of CD3+ cells in leukemic Vav1−/− mice (). As leukemic cells generally lacked TCRβ expression Vav1−/− mice (), we assessed whether these peripheral TCRβ− CD3- cells expressed CD4 or CD8 lineage markers. Indeed, there were more than 4-fold increases in both CD3-CD4+ and CD3-CD8+ cells in leukemic Vav1−/− mice (from > 5% to 15–20% of all splenocytes, ). Interestingly, the increase in these immature CD3- cells was modest in WT mice with leukemias (2–4% to approximately 8%; ). Furthermore, mature CD3+CD4+ and CD3+CD8+ were not significantly increased in leukemic WT mice and in fact, were decreased in the Vav1−/− mice. Thus, in Vav1−/− mice with leukemias, splenocytes as well as thymocytes harbor an immature CD3- T cell phenotype. Altogether, CD3+ leukemias were significantly decreased in Vav1−/− mice as compared with WT mice following infection with M-MuLV.
Figure 6. Analysis of splenocyte populations in M-MuLV infected mice. (A) Expression of CD3, CD19, CD4 and CD8 in splenocytes (Vav1+/+ control (n = 8); Vav1−/− control (n = 8); Vav1+/+ M-MuLV (n = 36); Vav1−/− M-MuLV (n = 46)) were assessed by flow cytometry and bar graphs presenting the mean percentages ± SEM of splenocytes expressing CD3 and CD19 are shown. B) Graphs presenting the mean percentages ± SEM of splenocytes expressing CD4 and CD8 in the absence or presence of surface CD3 are shown. Data were evaluated using Student’s t-test: * p < 0.05; **p < 0.01; ***p < 0.001.
Discussion
In this report, we investigated whether a protein that plays an essential role in lymphopoiesis also influences leukemogenesis. Vav1 strongly affects the T cell compartment without modulating other lineages and as such, it was of interest to selectively induce T cell leukemias. To this end, we used the M-MuLV retrovirus that lacks oncogenes and induces T cell lymphomas with a latency of 3–4 mo.Citation17 Here, we report that the presence of Vav1 regulates M-MuLV-induced T cell leukemogenesis. Vav1+/+ mice infected with M-MuLV develop the typical disease, characterized by a decrease in hematocrit, a modest enlargement of the thymus and a significant splenomegaly ( and and refs. Citation17,Citation20-Citation24). At the cellular level, leukemic cells predominantly expressed markers associated with T cell maturation such as CD3 and TCRβ ( and ). Notably, in the absence of Vav1, infection with M-MuLV resulted in leukemias characterized by higher hematocrits, massive thymus enlargement and a more modest splenomegaly. Furthermore, the leukemic phenotypes in the thymus as well as the periphery differed; with a significantly more immature phenotype in Vav1−/− as compared with WT mice.
Induction of tumors by M-MuLV presumably results from infection of a target cell and a series of subsequent tumor progression events. Target cells for M-MuLV are early cells committed to the T cell lineage and they differentiate to various stages in T-cell differentiation before the final transforming event.Citation21-Citation23 The identity of the infected cells during the early stages of leukemogenesis remains unknown. The most immature population in the thymus is the DN subset, which is subdivided into different stages: CD44+CD25- (DN1), the CD44+CD25+ (DN2) stage during which TCRβ rearrangements are initiated, the CD44-CD25+ (DN3) stage associated with initial CD3 expression and selection for productive TCRβ rearrangements, followed by the CD44-CD25- (DN4) stage of the TCRβ locus. The expression of a functional pre-TCR complexes leads to the DN4 stage and DP cells, expressing the CD4 and CD8 coreceptors, undergo TCRα rearrangements and express a mature TCRαβ complex. After positive and negative selection these cells become SP thymocytes with the maximal expression of CD3. The CD4+ thymocyte population increased in mice developing M-MuLV-induced leukemias. Importantly though, in Vav1−/− mice, leukemogenesis resulted in a massive accumulation in DN cells. Moreover, the DN population was altered in both mice strains, suggesting that these cells may represent a preleukemic state. As DN4 cells were the major DN population in infected Vav1−/− thymi, it is likely that leukemic cells overpassed the Vav1 requirement at the DN3 to DN4 transition () or alternatively, were infected at that stage as they show a proliferative burst.Citation30,Citation31
Because the thymocyte population that increased in lymphomagenic thymi was CD4+, we compared the expression of two T cell differentiation markers in leukemic Vav1+/+ and Vav1−/− CD4+ cells; CD3 and TCRβ. We found that CD4+ cells derived from leukemic Vav1−/− mice lacked expression of TCRβ ( and ), even if CD4 was expressed at high levels. In agreement with the thymus data, the vast majority of peripheral leukemic cells in Vav1−/− mice were CD3-. We favor a model in which, in the absence of Vav1, the pre-leukemic T cells fail to properly differentiate keeping their immature phenotype.
Vav1−/−, but not Vav2−/− or Vav3−/−, mice are prone to developing lymphoblastic lymphoma-like tumors during aging.Citation16 It is possible that in old animals, exhaustion of T cell activity and increased pressure for T cells to divide could be responsible for the increase in tumorigenesis. However, the phenotype of “spontaneous” lymphomas in aging Vav1−/− mice is similar to that detected in aging wt mice. This might suggest that it is the increased homeostatic-drive proliferation occurring in lymphopenic Vav1−/− mice that contributes to leukemia formation in a spontaneous setting. In contrast, under the conditions reported here, M-MuLV clearly favored the transformation of a more immature T cell precursor. Compare with our results (), aging leukemic Vav1−/− mice do not show massive thymic enlargement.Citation16 It could be that initial leukemic cells are produced in a more mature phenotype in aging mice, which do not localize in the thymus. An alternative explanation is that aging thymi lose partially their functions,Citation32 allowing tumor cells to exit the thymus even at the first stages of the disease.
Vav1 is misssexpressed and plays a significant role in different kind of non-hematopoietic tumors,Citation5,Citation6 including melanoma cell invasion.Citation7 However, gain-of-function mutations in the Vav1 gene have not yet been found in human tumors of hematopoietic origin. Perhaps primary cells select against such mutations. In fact, a recent study on mice has shown that oncogenic Vav1 expression inhibits hematopoietic stem cell engraftment and cell expansion in vitro by inducing apoptosis.Citation33
Studies of M-MuLV-induced leukemia have provided insights into the multistep process of leukemogenesis. The fact that the disease occurs with a moderate, predictable latency has allowed identification of the virological and physiological steps that occur during preleukemic stages and during tumor progression. Some of the principles uncovered are likely to be applicable to the development of neoplasms in other species, including humans. This model is complex and often involves activation of more than a specific oncogenic pathway. Human T cell leukemia virus (HTLV-1), the etiologic agent of adult T cell leukemia (ATL), can be transmitted by breastfeeding but only leads to the development of the disease in 2–5% of the infected infants and only after latency periods of 20 to 60 y.Citation34 Transformed ATL cells are generally CD4+ and it is interesting to note that in our WT mice, the majority of M-MuLV-induced leukemias were of the CD4 phenotype. Some ATL, as well as mice harboring the Tax gene of HTLV-1, present with an immature CD4-CD8- phenotype.Citation35 It will be of interest to assess Vav1 expression in these leukemias, which could correlate with the developmental status of the leukemic cells.
All together our data suggest that the proteins involved in T cell differentiation also regulate the development of T cell leukemias. Induction of tumors by a retrovirus results from infection of a target cell and a series of subsequent tumor progression events. The data presented here suggest that the target cells wherein M-MuLV promotes tumor formation differ in the presence and absence of Vav1. In Vav1 deficient mice, impaired T cell development during the selection processes favors the emergence of a different tumor phenotype.
Materials and Methods
Mice
Vav1−/− mice in the 129 SV background, a generous gift from Dr. V. Tybulewicz, have been previously describedCitation12 and were maintained under the same conditions as 129 SV mice. All animal experiments were performed according to the guidelines and regulations of the Centre National de la Recherche Scientifique.
Virus inoculation and clinical evaluation of mice
Vav1−/−, Vav1+/− and 129 SV mice, 18 to 72 h old, were inoculated intraperitoneally with 105 foci forming units (FFU) of M-MuLV in 50 μL as previously described.Citation20 Mice were monitored for gross organ enlargement by palpation under Forene® (isofurane, Abbott France) anesthesia. Hematocrits, expressed as the percentage of erythrocytes in the blood volume, were measured in retroorbital vein blood samples collected in heparinized capillary tubes. Fifty days after infection, hematocrits were monitored regularly at approximately 20-d intervals. After sacrifice of moribund animals, spleens were weighed and thymic size was scored from normal, to clearly enlarge (medium), to invading the whole thoracic cavity (large).
Immunophenotyping and flow cytometry analyses
Splenocytes and thymocytes were stained with the appropriate conjugated αCD8, αCD4, αCD3, αCD25, αCD44, Ki-67, TCRβ (PharMingen), αCD3 and αCD19 (Immunotech) mouse monoclonal Abs, as indicated. Stained cells were analyzed by flow cytometry using a FACSCalibur (Becton Dickinson) and analyses were performed with CellQuest (Becton Dickinson) or FlowJo (Tree Star) software as previously described.Citation36,Citation37
Statistical analysis
All results were analyzed by an unpaired Student’s t-test.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the members of the animal facility at the IGMM. This work was supported by the program “Chercheur d’avenir” from the Region Languedoc-Rousillon (MV), a scientific program from the “Communauté de Travail des Pyrénées” (CTPP10/09 to MV), the Association pour la Recherche contre le Cancer (MV and NT), the Fondation pour la Recherche Medicale (MV), a grant FEDER Objectif competitivite (MV), a grant from European Community Program SUDOE (MV) and the FP7 integrated project grant ATTACK (NT) and a fellowship from “La Ligue Nationale Contre le Cancer (S.K.).
Notes
† These authors contributed equally to this work
References
- Sahai E, Marshall CJ. RHO-GTPases and cancer. Nat Rev Cancer 2002; 2:133 - 42; http://dx.doi.org/10.1038/nrc725; PMID: 12635176
- Bustelo XR. Regulatory and signaling properties of the Vav family. Mol Cell Biol 2000; 20:1461 - 77; http://dx.doi.org/10.1128/MCB.20.5.1461-1477.2000; PMID: 10669724
- Katzav S, Martin-Zanca D, Barbacid M. vav, a novel human oncogene derived from a locus ubiquitously expressed in hematopoietic cells. EMBO J 1989; 8:2283 - 90; PMID: 2477241
- Lazer G, Katzav S. Guanine nucleotide exchange factors for RhoGTPases: good therapeutic targets for cancer therapy?. Cell Signal 2011; 23:969 - 79; http://dx.doi.org/10.1016/j.cellsig.2010.10.022; PMID: 21044680
- Fernandez-Zapico ME, Gonzalez-Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, et al. Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 2005; 7:39 - 49; http://dx.doi.org/10.1016/j.ccr.2004.11.024; PMID: 15652748
- Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan-Ramu N, Katzav S. The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J Pathol 2003; 199:526 - 33; http://dx.doi.org/10.1002/path.1314; PMID: 12635144
- Bartolomé RA, Molina-Ortiz I, Samaniego R, Sánchez-Mateos P, Bustelo XR, Teixidó J. Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane-type matrix metalloproteinase-dependent melanoma cell invasion. Cancer Res 2006; 66:248 - 58; http://dx.doi.org/10.1158/0008-5472.CAN-05-2489; PMID: 16397238
- Poppe D, Tiede I, Fritz G, Becker C, Bartsch B, Wirtz S, et al. Azathioprine suppresses ezrin-radixin-moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol 2006; 176:640 - 51; PMID: 16365460
- Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, et al. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest 2003; 111:1133 - 45; PMID: 12697733
- Tybulewicz VL. Vav-family proteins in T-cell signalling. Curr Opin Immunol 2005; 17:267 - 74; http://dx.doi.org/10.1016/j.coi.2005.04.003; PMID: 15886116
- Tybulewicz VL, Ardouin L, Prisco A, Reynolds LF. Vav1: a key signal transducer downstream of the TCR. Immunol Rev 2003; 192:42 - 52; http://dx.doi.org/10.1034/j.1600-065X.2003.00032.x; PMID: 12670394
- Villalba M, Bi K, Hu J, Altman Y, Bushway P, Reits E, et al. Translocation of PKC[theta] in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J Cell Biol 2002; 157:253 - 63; http://dx.doi.org/10.1083/jcb.200201097; PMID: 11956228
- Villalba M, Bi K, Rodriguez F, Tanaka Y, Schoenberger S, Altman A. Vav1/Rac-dependent actin cytoskeleton reorganization is required for lipid raft clustering in T cells. J Cell Biol 2001; 155:331 - 8; http://dx.doi.org/10.1083/jcb.200107080; PMID: 11684704
- Holsinger LJ, Graef IA, Swat W, Chi T, Bautista DM, Davidson L, et al. Defects in actin-cap formation in Vav-deficient mice implicate an actin requirement for lymphocyte signal transduction. Curr Biol 1998; 8:563 - 72; http://dx.doi.org/10.1016/S0960-9822(98)70225-8; PMID: 9601640
- Fischer KD, Kong YY, Nishina H, Tedford K, Marengère LE, Kozieradzki I, et al. Vav is a regulator of cytoskeletal reorganization mediated by the T-cell receptor. Curr Biol 1998; 8:554 - 62; http://dx.doi.org/10.1016/S0960-9822(98)70224-6; PMID: 9601639
- Ruiz S, Santos E, Bustelo XR. The use of knockout mice reveals a synergistic role of the Vav1 and Rasgrf2 gene deficiencies in lymphomagenesis and metastasis. PLoS ONE 2009; 4:e8229; http://dx.doi.org/10.1371/journal.pone.0008229; PMID: 20011522
- Fan H. Leukemogenesis by Moloney murine leukemia virus: a multistep process. Trends Microbiol 1997; 5:74 - 82; http://dx.doi.org/10.1016/S0966-842X(96)10076-7; PMID: 9108934
- West AG, Fraser P. Remote control of gene transcription. Hum Mol Genet 2005; 14:Spec No 1 R101 - 11; http://dx.doi.org/10.1093/hmg/ddi104; PMID: 15809261
- Uren AG, Kool J, Berns A, van Lohuizen M. Retroviral insertional mutagenesis: past, present and future. Oncogene 2005; 24:7656 - 72; http://dx.doi.org/10.1038/sj.onc.1209043; PMID: 16299527
- Garaude J, Kaminski S, Charni S, Aguilò JI, Jacquet C, Plays M, et al. Impaired anti-leukemic immune response in PKCtheta-deficient mice. Mol Immunol 2008; 45:3463 - 9; http://dx.doi.org/10.1016/j.molimm.2008.03.016; PMID: 18462800
- Lazo PA, Klein-Szanto AJ, Tsichlis PN. T-cell lymphoma lines derived from rat thymomas induced by Moloney murine leukemia virus: phenotypic diversity and its implications. J Virol 1990; 64:3948 - 59; PMID: 2196385
- Clark SS, Chen E, Fizzotti M, Witte ON, Malkovska V. BCR-ABL and v-abl oncogenes induce distinct patterns of thymic lymphoma involving different lymphocyte subsets. J Virol 1993; 67:6033 - 46; PMID: 8396667
- Ott DE, Keller J, Sill K, Rein A. Phenotypes of murine leukemia virus-induced tumors: influence of 3′ viral coding sequences. J Virol 1992; 66:6107 - 16; PMID: 1326661
- Audit M, Déjardin J, Hohl B, Sidobre C, Hope TJ, Mougel M, et al. Introduction of a cis-acting mutation in the capsid-coding gene of moloney murine leukemia virus extends its leukemogenic properties. J Virol 1999; 73:10472 - 9; PMID: 10559365
- Storch TG, Arnstein P, Manohar V, Leiserson WM, Chused TM. Proliferation of infected lymphoid precursors before Moloney murine leukemia virus-induced T-cell lymphoma. J Natl Cancer Inst 1985; 74:137 - 43; PMID: 3871491
- Sen-Majumdar A, Weissman IL, Hansteen G, Marian J, Waller EK, Lieberman M. Radiation leukemia virus-induced thymic lymphomas express a restricted repertoire of T-cell receptor V beta gene products. J Virol 1994; 68:1165 - 72; PMID: 8289345
- Raberger J, Boucheron N, Sakaguchi S, Penninger JM, Ellmeier W. Impaired T-cell development in the absence of Vav1 and Itk. Eur J Immunol 2008; 38:3530 - 42; http://dx.doi.org/10.1002/eji.200838388; PMID: 19009524
- Doody GM, Bell SE, Vigorito E, Clayton E, McAdam S, Tooze R, et al. Signal transduction through Vav-2 participates in humoral immune responses and B cell maturation. Nat Immunol 2001; 2:542 - 7; http://dx.doi.org/10.1038/88748; PMID: 11376342
- Tedford K, Nitschke L, Girkontaite I, Charlesworth A, Chan G, Sakk V, et al. Compensation between Vav-1 and Vav-2 in B cell development and antigen receptor signaling. Nat Immunol 2001; 2:548 - 55; http://dx.doi.org/10.1038/88756; PMID: 11376343
- Tourigny MR, Mazel S, Burtrum DB, Petrie HT. T cell receptor (TCR)-beta gene recombination: dissociation from cell cycle regulation and developmental progression during T cell ontogeny. J Exp Med 1997; 185:1549 - 56; http://dx.doi.org/10.1084/jem.185.9.1549; PMID: 9151892
- Hoffman ES, Passoni L, Crompton T, Leu TM, Schatz DG, Koff A, et al. Productive T-cell receptor beta-chain gene rearrangement: coincident regulation of cell cycle and clonality during development in vivo. Genes Dev 1996; 10:948 - 62; http://dx.doi.org/10.1101/gad.10.8.948; PMID: 8608942
- Arnold CR, Wolf J, Brunner S, Herndler-Brandstetter D, Grubeck-Loebenstein B. Gain and loss of T cell subsets in old age--age-related reshaping of the T cell repertoire. J Clin Immunol 2011; 31:137 - 46; http://dx.doi.org/10.1007/s10875-010-9499-x; PMID: 21243520
- Gu Y, Siefring JE, Wang L, Chae HD, Bailey JR, Zheng Y. Oncogenic Vav1 induces Rac-dependent apoptosis via inhibition of Bcl-2 family proteins and collaborates with p53 deficiency to promote hematopoietic progenitor cell proliferation. Oncogene 2006; 25:3963 - 72; http://dx.doi.org/10.1038/sj.onc.1209427; PMID: 16474842
- Takatsuki K, Yamaguchi K, Kawano F, Hattori T, Nishimura H, Tsuda H, et al. Clinical diversity in adult T-cell leukemia-lymphoma. Cancer Res 1985; 45:Suppl 4644s - 5s; PMID: 2861896
- Hasegawa H, Sawa H, Lewis MJ, Orba Y, Sheehy N, Yamamoto Y, et al. Thymus-derived leukemia-lymphoma in mice transgenic for the Tax gene of human T-lymphotropic virus type I. Nat Med 2006; 12:466 - 72; http://dx.doi.org/10.1038/nm1389; PMID: 16550188
- Charni S, Aguilo JI, Garaude J, de Bettignies G, Jacquet C, Hipskind RA, et al. ERK5 knockdown generates mouse leukemia cells with low MHC class I levels that activate NK cells and block tumorigenesis. J Immunol 2009; 182:3398 - 405; http://dx.doi.org/10.4049/jimmunol.0803006; PMID: 19265117
- Charni S, de Bettignies G, Rathore MG, Aguiló JI, van den Elsen PJ, Haouzi D, et al. Oxidative phosphorylation induces de novo expression of the MHC class I in tumor cells through the ERK5 pathway. J Immunol 2010; 185:3498 - 503; http://dx.doi.org/10.4049/jimmunol.1001250; PMID: 20729331