Abstract
Low efficacy of peptide vaccines limits their potential application. We developed a powerful strategy to produce epitope-specific antibodies using peptides. Immunization with novel formula into mice showed target-specific prophylactic and therapeutic effects against tumors. Our strategy will be useful for rapid eiptope screening, therapeutic antibody production and cancer vaccine development.
Keywords: :
Innate immunity and adaptive immunity synergistically protect our bodies from various non-self pathogens such as bacteria, parasites, fungi, and viruses. These system also recognize cancer cells as an altered-self and remove them. Adaptive immunity exhibits specificity against antigens through antigen-specific receptors and antibodies. Epitope-based peptide vaccines have been extensively studied in various animal models for the past 30 y and have proved to be pivotal for inducing and regulating immune responses through their binding ability to B cell receptors and MHC as B-cell epitopes and T-cell epitopes. Peptides are easy to synthesize in a short time and the cost is inexpensive. Therefore, peptide vaccines are potentially useful as prophylaxis for cancers and infectious diseases such as influenza virus, malaria, hepatitis B, and HIV.Citation1 However, the efficacy of peptide vaccines is limited in the treatment of patients. To maximize the magnitude of peptide-driven immune responses, we used a strategy of stimulating the innate immunity with CpG-DNA and facilitated delivery of vaccines using liposomes.Citation2,Citation3
CpG-DNA represents synthetic oligodeoxynucleotides (ODNs) and bacterial DNA containing unmethylated CpG dinucleotides flanked by specific base sequences. Because CpG DNA has significant immunomodulatory effects on B lymphocytes, macrophages, dendritic cells, and natural killer cells, it has gained attention for its potential use as an immune adjuvant. Many investigators have utilized phosphorothioate-modified types of CpG-DNA (PS-ODN), which are resistant to nuclease activity and can be efficiently delivered into cells.Citation4 However, PS-ODN induces backbone-related side effects, such as transient splenomegaly, arthritis, and PS-ODN-specific IgM production in PS-ODN-treated mice.Citation5 Therefore, investigators, including us, have developed phosphodiester bond CpG-DNA (PO-ODN) as a natural counterpart of PS-ODN to induce optimal innate immune responses. We screened natural PO-ODN with immunomodulatory activity from Mycobacterium bovis genomic DNA and isolated a potent PO-ODN, namely MB-ODN 4531(O), which contains three CpG motifs and functions as a powerful adjuvant without causing severe side effects in mice.Citation6 In contrast to PS-ODN, PO-ODN shows an immunomodulatory effect only in mouse cells and not in human cells.Citation7 Liposomes are potent vehicles for delivering antigens to antigen presenting cells and are known to enhance antibody production and cytotoxic T lymphocyte (CTL) responses.Citation8 To enhance the potency of PO-ODN in human cells, we tested several different formulas of liposome complexes and found a special composition of choice: phosphatidyl-β-oleoyl-γ-palmitoyl ethanolamine (DOPE)/cholesterol hemisuccinate (CHEMS) (1:1 ratio). The natural CpG-DNA, in combination with the DOPE/CHEMS complex (Lipoplex(O)), increased expression of cytokines such as IL-6, IL-12, and IFN-γ in human cells, as well as in mouse cells, through improved intracellular uptake of CpG-DNA and TLR9/MyD88-mediated cellular activation. Levels of cytokine expression in mouse serum were also elevated by Lipolex(O) in TLR9-dependent and CpG-sequence dependent manners. Furthermore, Lipoplex(O) had an adjuvant activity when proteins such as hen egg lysozyme and ovalbumin were used as antigens in mice. Therefore, we next tried to apply Lipoplex(O) for a synthetic peptide-based B cell epitope screening and antibody production using peptides as an antigen.Citation2
We predicted candidate B cell epitopes from a cancer specific antigen and viral antigens using computer algorithms and injected each epitope along with Lipoplex(O), into mice; we then checked for the production of epitope-specific antibodies.Citation2,Citation3,Citation9 The results revealed that our strategy is very useful for the selection of potent B cell epitopes. To further evaluate our strategy in detail, we focused on studying the use of a B cell epitope peptide (TM4SF5R2–3) that we screened from hepatocellular carcinoma (HCC)-specific transmembrane 4 superfamily member 5 (TM4SF5) protein.Citation10 Complexes of peptide and Lipoplex(O) significantly enhanced peptide-specific IgG production depending on TLR9, CD4+ T cell, MHC-II, and Th1 cell differentiation. Considering that we are using only B cell epitope without any carrier, the involvement of the CD4+ T cell and MHC-II is a very interesting characteristic of the response. Even though we do not know the exact mechanism involved in this process, our powerful strategy to produce epitope-specific antibodies without the need of a carrier protein has a clear advantage (). Monoclonal antibody produced by immunization with a complex consisting of TM4SF5R2–3 and Lipoplex(O) inhibited the growth of HCC cells expressing the antigen. Furthermore, immunization with a complex consisting of TM4SF5R2–3 and Lipoplex(O) protected mice from mouse HCC cell implantation in a TLR9-dependent manner. Preimmunization with our vaccine induced robust production of peptide-specific antibodies after cancer cell challenge and significantly suppressed tumor growth (tumor weight 2.5 g vs. 0.25 g), proving its prophylactic effect. Immunization after cancer cell implantation also significantly suppressed tumor growth (0.8 g vs. 0.25 g) and increased survival rate (0% vs. 100% at 100 d after cancer cell implantation) revealing the method's therapeutic effect. Therefore, we conclude that our peptide vaccine works successfully without apparent side effects in the mouse cancer model.Citation3 Because functional antibodies produced using this strategy can be applied as therapeutics for cancers after the humanization process, the experiment is ongoing. Further investigation of the functional mechanism of our novel vaccine will shed more light on the rationale of our vaccine.
Figure 1. Composition of novel peptide vaccines, some key mechanisms, evaluation in mice, and their possible application. Our peptide vaccine is a complex consisting of B cell epitope peptide, natural phosphodiester CpG-DNA, and DOPE:CHEMS liposome mixture. Immunization with the peptide vaccine in mice induces production of epitope-specific antibodies. Based on our experimental results, this process involves CpG-DNA, TLR9, MHC-II, CD4 T cells, and Th1 differentiation. Further investigation of the functional mechanism will give us a better understanding of the powerful efficacy of our novel peptide vaccine. We confirmed prophylactic and therapeutic effects with peptide vaccine targeting a cancer-specific antigen in the mouse hepatocellular cancer (HCC) model. We believe that this strategy can be widely used in rapid epitope screening, therapeutic antibody development, cancer vaccines, and defense against infectious diseases.
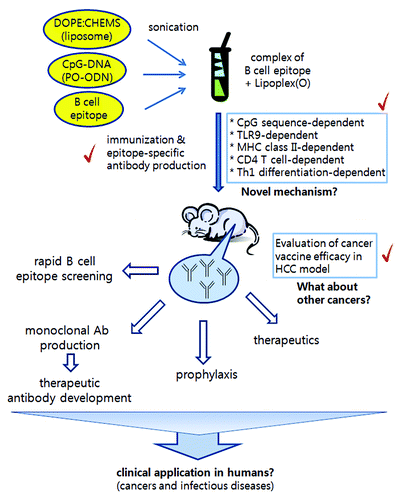
We believe that our strategy will contribute to a more effective defense against life-threatening diseases. Recently, the appearance of novel viruses and the globalization of the world have created extended threats to human beings. Our novel strategy can be promptly used in rapid screening of potent B cell epitopes and can enable timely defenses against pandemics of infectious diseases. It can potentially be applied for the development of therapeutic antibodies against exposure to bioterrorism agents. Evaluation of our vaccine in cancers other than HCC will further expand applications.
References
- Bijker MS, Melief CJ, Offringa R, van der Burg SH. Design and development of synthetic peptide vaccines: past, present and future. Expert Rev Vaccines 2007; 6:591 - 603; http://dx.doi.org/10.1586/14760584.6.4.591; PMID: 17669012
- Kim D, Kwon S, Rhee JW, Kim KD, Kim YE, Park CS, et al. Production of antibodies with peptide-CpG-DNA-liposome complex without carriers. BMC Immunol 2011; 12:29; http://dx.doi.org/10.1186/1471-2172-12-29; PMID: 21592346
- Kwon S, Kim D, Park BK, Cho S, Kim KD, Kim YE, et al. Prevention and therapy of hepatocellular carcinoma by vaccination with TM4SF5 epitope-CpG-DNA-liposome complex without carriers. PLoS One 2012; 7:e33121; http://dx.doi.org/10.1371/journal.pone.0033121; PMID: 22427965
- Krieg AM. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov 2006; 5:471 - 84; http://dx.doi.org/10.1038/nrd2059; PMID: 16763660
- Kim D, Rhee JW, Kwon S, Sohn WJ, Lee Y, Kim DW, et al. Immunostimulation and anti-DNA antibody production by backbone modified CpG-DNA. Biochem Biophys Res Commun 2009; 379:362 - 7; http://dx.doi.org/10.1016/j.bbrc.2008.12.063; PMID: 19103173
- Lee KW, Jung J, Lee Y, Kim TY, Choi SY, Park J, et al. Immunostimulatory oligodeoxynucleotide isolated from genome wide screening of Mycobacterium bovis chromosomal DNA. Mol Immunol 2006; 43:2107 - 18; http://dx.doi.org/10.1016/j.molimm.2005.12.004; PMID: 16442622
- Liang H, Nishioka Y, Reich CF, Pisetsky DS, Lipsky PE. Activation of human B cells by phosphorothioate oligodeoxynucleotides. J Clin Invest 1996; 98:1119 - 29; http://dx.doi.org/10.1172/JCI118894; PMID: 8787674
- Bhowmick S, Mazumdar T, Sinha R, Ali N. Comparison of liposome based antigen delivery systems for protection against Leishmania donovani. J Control Release 2010; 141:199 - 207; http://dx.doi.org/10.1016/j.jconrel.2009.09.018; PMID: 19818373
- Kim D, Kwon HJ, Lee Y. Activation of Toll-like receptor 9 and production of epitope specific antibody by liposome-encapsulated CpG-DNA. BMB Rep 2011; 44:607 - 12; http://dx.doi.org/10.5483/BMBRep.2011.44.9.607; PMID: 21944255
- Lee SA, Lee SY, Cho IH, Oh MA, Kang ES, Kim YB, et al. Tetraspanin TM4SF5 mediates loss of contact inhibition through epithelial-mesenchymal transition in human hepatocarcinoma. J Clin Invest 2008; 118:1354 - 66; http://dx.doi.org/10.1172/JCI33768; PMID: 18357344