Abstract
Although it is recognized that immune function is modulated by androgen ablation therapy for prostate cancer, the long-term consequences are not completely understood. We recently showed that both effector and inhibitory immune mechanisms are amplified by androgen ablation, providing one explanation for only transient increases in immune function after castration.
Androgen ablation is a first-line therapy for advanced prostate cancer, and results in apoptotic death of the primary tumor. Studies of immune function early after androgen ablation showed that hormone removal increased T-cell responses to prostate tumor antigens.Citation1,Citation2 However, immune augmentation must be transient, since castration-resistant disease recurs, months to years after treatment of the primary tumor. Thus, understanding the long-term impact of androgen ablation/blockade on the immune system will permit the development of more effective immune therapies for advanced prostate cancer.
The mechanisms for increased T-cell responses after castration are not completely understood. Androgen ablation increases thymus size and peripheral T-cell numbers (reviewed in ref. Citation3), suggesting that heightened responses may be due to greater numbers of naive T cells capable of responding to tumor antigens. Subsequent studies in autoimmune disease models have shown that hormone blockade/removal ameliorates autoimmune disease, suggesting that inhibitory immune mechanisms that control effector responses may also be increased (reviewed in ref. Citation4).
In the context of prostate cancer, pre-clinical and clinical studies have shown that CD4+ and CD8+ T-cell responses are increased early after androgen ablation.Citation1 Functional CD8+ cytolytic T cells are required for the rejection of solid tumors. In a murine model of endogenous prostate cancer generated by prostate-specific deletion of the Pten tumor suppressor gene, we observed that functional CD8+ T-cell numbers were increased at an early time point after surgical castration, but declined rapidly to pre-castration levels.Citation5
Building on these data and the observations of ameliorated autoimmune disease after androgen ablation, we hypothesized that immune inhibitory mechanisms must be amplified after castration as well. In our recent work, we found that the numbers of CD4+CD25+FoxP3+ regulatory T cells (Tregs) were amplified in the prostate draining lymph node and spleen of castrated Pten −/− mice compared with sham-castrated Pten−/− mice.Citation6 This increase was observed at an early time point (2.5 weeks) post-castration, when functional GzB+/CD8+ T cells in the prostate tumor were also increased in number. To determine whether increased Tregs affected CD8+ T-cell responses to a defined tumor antigen, we immunized Pten−/− mice with the model tumor cell line, UV8101-RE. Heightened responses to this antigen were only observed when Tregs were also depleted together with castration. Increased functional antigen-specific CD8+ T cells were maintained for several weeks (5 weeks post-castration) in the LN and spleen, demonstrating that Treg depletion both increased and sustained effector T-cell function. These data suggest that increased Tregs may prevent the maintenance of CD8+ T-cell responses to prostate tumor antigens shed by the dying primary prostate tumor, and may be one mechanism responsible for only transient increase in effector function after castration.
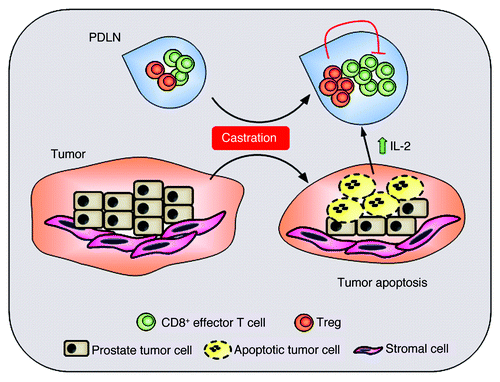
It is presumed that the dying prostate epithelial cells shed previously sequestered tumor antigens which then activate CD8+ and CD4+ T cells, leading to secretion of effector cytokines such as interleukin-2 (IL-2) by the T cells. In addition to supporting effector T-cell proliferation and differentiation, IL-2 is the signature cytokine required for the maintenance and expansion of Tregs.Citation7 We showed that in vivo blockade of IL-2 together with castration of Pten−/− mice prevented Treg expansion. Together, our results suggest the following model (): surgical castration causes apoptosis of hormone dependent cancerous prostate epithelium, leading to processing and presentation of shed tumor antigens, and amplification of functional CD8+ T cells within the tumor. Increased IL-2 produced by the activated effector T cells leads to expansion of Tregs, which then inhibit CD8+ T-cell function.Citation8,Citation9 This paracrine loop is at least partially responsible for prostate cancer progression after castration. It is possible that androgen ablation may also change Treg homeostasis through modulation of thymic T-cell development, contributing to Treg expansion after immunization.
Figure 1. Proposed model for amplification of Tregs after castration. Surgical castration induces apoptotic death of cancerous prostate epithelium. Antigens shed by the dying prostate tumor elicit effector CD8+ T-cell responses, which induce production of IL-2 by effector T cells. Preferential consumption of IL-2 by Tregs leads to Treg expansion and subsequent inhibition of CD8+ T-cell function in the prostate draining lymph nodes (PDLN).
We depleted Tregs by administration of anti-CD25 antibody 2 d prior to castration. A limitation of this therapy is the potential collateral elimination of CD25+ effector T cells. In our system, however, anti-CD25 treatment augmented CD8+ effector cell function. We speculate that the availability of IL-2 as a result of Treg depletion heightens effector T-cell proliferation, compensating for an initial depletion of CD25+ effector T cells. Alternately, only CD25hi T cells, which may be predominantly Tregs, are depleted by anti-CD25 administration.Citation10 Importantly, Tregs were amplified after castration only when immune responses against tumor antigens were also induced, and not when wild-type animals were castrated alone, further strengthening the suggestion that increased IL-2 caused the paradoxical response.
Our results imply that other treatments such as chemotherapy or radiation therapy, which also induce massive tumor cell death, can expand both effector T cells and Tregs. Treg depletion prior to or along with tumoridical therapy may augment effector anti-tumor immune responses, preventing tumor progression and development of metastatic disease.
References
- Mercader M, Bodner BK, Moser MT, Kwon PS, Park ES, Manecke RG, et al. T cell infiltration of the prostate induced by androgen withdrawal in patients with prostate cancer. Proc Natl Acad Sci U S A 2001; 98:14565 - 70; http://dx.doi.org/10.1073/pnas.251140998; PMID: 11734652
- Drake CG, Doody AD, Mihalyo MA, Huang CT, Kelleher E, Ravi S, et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 2005; 7:239 - 49; http://dx.doi.org/10.1016/j.ccr.2005.01.027; PMID: 15766662
- Aragon-Ching JB, Williams KM, Gulley JL. Impact of androgen-deprivation therapy on the immune system: implications for combination therapy of prostate cancer. Front Biosci 2007; 12:4957 - 71; http://dx.doi.org/10.2741/2441; PMID: 17569623
- Ansar Ahmed S, Penhale WJ, Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol 1985; 121:531 - 51; PMID: 3907369
- Akins EJ, Moore ML, Tang S, Willingham MC, Tooze JA, Dubey P. In situ vaccination combined with androgen ablation and regulatory T-cell depletion reduces castration-resistant tumor burden in prostate-specific pten knockout mice. Cancer Res 2010; 70:3473 - 82; http://dx.doi.org/10.1158/0008-5472.CAN-09-2490; PMID: 20406970
- Tang S, Moore ML, Grayson JM, Dubey P. Increased CD8+ T-cell Function following Castration and Immunization Is Countered by Parallel Expansion of Regulatory T Cells. Cancer Res 2012; 72:1975 - 85; http://dx.doi.org/10.1158/0008-5472.CAN-11-2499; PMID: 22374980
- Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity 2010; 33:153 - 65; http://dx.doi.org/10.1016/j.immuni.2010.08.004; PMID: 20732639
- McNally A, Hill GR, Sparwasser T, Thomas R, Steptoe RJ. CD4+CD25+ regulatory T cells control CD8+ T-cell effector differentiation by modulating IL-2 homeostasis. Proc Natl Acad Sci U S A 2011; 108:7529 - 34; http://dx.doi.org/10.1073/pnas.1103782108; PMID: 21502514
- Kastenmuller W, Gasteiger G, Subramanian N, Sparwasser T, Busch DH, Belkaid Y, et al. Regulatory T cells selectively control CD8+ T cell effector pool size via IL-2 restriction. J Immunol 2011; 187:3186 - 97; http://dx.doi.org/10.4049/jimmunol.1101649; PMID: 21849683
- Dannull J, Su Z, Rizzieri D, Yang BK, Coleman D, Yancey D, et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J Clin Invest 2005; 115:3623 - 33; http://dx.doi.org/10.1172/JCI25947; PMID: 16308572