Abstract
Augmented numbers of regulatory T cells contribute to the overall immunosuppression in tumor patients. Interleukin-2 has been widely used in the clinics in anticancer therapy, yet evidence has accumulated that the major drawback, limiting clinical efficacy, is the expansion of regulatory T cells, which aggravates immunosuppression.
Keywords: :
Interleukin-2 (IL-2) has been identified more than 30 years ago and primarily been described as a factor acting on conventional T cells to promote their activation and proliferation.Citation1 Due to its T-cell activating and expanding properties, IL-2 has been introduced early into the immunotherapy of cancer patients, either as a single agent or in combination with other cytokines or chemotherapy.Citation1 However, over the last several years it has become clear that IL-2 not only has beneficial properties, but also can expand regulatory T (Treg) cells.Citation1
Treg cells are involved in self-tolerance, immune homeostasis, prevention of autoimmunity, and suppression of immunity to pathogens.Citation2 The forkhead transcription factor FOXP3 is essential for Treg-cell development and function, as mutations in FOXP3 cause autoimmunity in mice and the IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome in humans.Citation2
In tumor-bearing individuals, Treg cells are increased in numbers both in the local tumor microenvironment and in the peripheral blood, and can contribute to the overall immunosuppression.Citation3 In several human tumors, Treg cells even have prognostic significance and their abundance can be correlated with the stage of disease.Citation3 Depletion of Treg cells has been suggested as a therapeutic option, with early clinical trials using an IL-2 immunotoxin showing promising results.Citation3
One important and yet unresolved aspect in tumor immunology is how and where Treg cells in tumor patients develop. Peripheral induction is one possibility, whereby tumor antigen-specific T cells might be converted into FOXP3-expressing Treg cells with suppressive functions within the tumor microenvironment. An alternative scenario would be the accumulation of Treg cells generated in the thymus, which would be attracted to the tumor site by specific factors such as chemokines.
Several studies have assessed the abundance and function of human Treg cells after IL-2 administration, and the overall consensus was that IL-2 augments their frequency.Citation4-Citation6 Possible explanations for such an increase were peripheral expansion of Treg cells but also altered migratory activity.Citation7 However, none of these studies assessed how IL-2 influenced the thymic output of Treg cells and whether this would interfere with efficient antitumor immune responses.
In a recent study,Citation8 we investigated how IL-2 treatment influences Treg-cell numbers and function in colorectal cancer patients undergoing a combined immunochemotherapy. We observed increased levels of Treg cells, as determined by a combined staining for CD4, FOXP3, and CD25 in the peripheral blood of these patients at the start of therapy, confirming previously published observations.Citation3 These cells expressed typical Treg-cell markers including CTLA-4 and GITR and had normal immunosuppressive functions. Next, we assessed the influence of IL-2 on the number of total Treg cells after completion of therapy, finding an expansion of the pool of Treg cells in IL-2 treated patients. This is in line with previously published studies, which also reported elevated numbers of Treg cells after treatment with IL-2.Citation4-Citation6
Similar to conventional T cells, Treg cells can be distinguished into memory and naïve subsets, according to the surface expression of CD45RA. Valmori et al. were the first to report that a subset of naïve Treg cells exists that is anergic following stimulation in the absence of IL-2, exerts ex vivo cell-cell contact-mediated suppressor functions yet proliferates in response to stimulation with autologous antigen-presenting cells.Citation9 These observations indicate that a high proportion of these cells have self-reactive T-cell receptors and hence that they are derived from the thymic Treg-cell compartment.Citation9 The relationship between memory and naïve Treg cells was further delineated in humans using genomic and functional approaches by Miyara et al., who clearly established that naïve Treg cells are an important subpopulation of human FOXP3+ Treg cells.Citation10 Using a similar gating strategy, which included the assessment of CCR7 expression to distinguish between central- and effector-memory Treg cells, we could detect an increase in naïve Treg cells before the initiation of immunotherapy. This increase in naïve Treg cells was even more pronounced after IL-2 administration and assessment of their suppressive function showed immunosuppressive activity comparable to that of memory Treg cells. One approach to determine the vicinity of T cells to the thymus is to determine the number of T-cell receptor excision circles (TRECs). Assessing TRECs in sorted naïve Treg cells from healthy donors and patients, before and after therapy, indicated that naïve Treg cells are enriched in thymus-derived Treg cells even before therapy, but particularly after the administration of IL-2, suggesting that IL-2 primarily acts on the thymus to produce additional Treg cells that join those already present in the tumor microenvironment and the peripheral blood of these patients.
To substantiate this observation, we administered IL-2 in a murine model system and could show that the IL-2 treatment results in an expansion of naïve Treg cells in all immunological cell compartments. This was mainly due to an increased thymic output, as assessed by analyzing TRECs in the sorted naïve Treg cells from these animals.
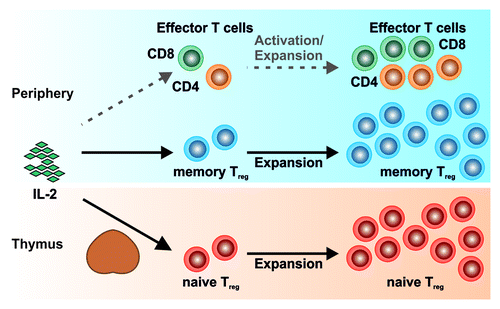
Taken together, our data supports an overall increase in Treg cells in tumor patients with an expansion of newly generated naïve Treg cells post IL-2 therapy as a major mechanism of the Treg-cell expansion in IL-2 treated tumor patients (). This finding has implications for the future direction on how to target Treg cells in tumor patients. Depletion of Treg cells, e.g., by the administration of Treg-cell targeting antibodies or immunotoxins, will only result in a short-term depletion of peripheral Treg cells. Long-term reduction of Treg cells will warrant therapeutic strategies reducing the thymic output of Treg cells, thus properly circumventing their immunosuppressive functions in tumor patients.
Figure 1. Interleukin 2 administration results in a preferential expansion of regulatory T (Treg) cells. Administration of interleukin-2 to tumor patients only results in a weak activation and expansion of potentially tumor-counteracting effector T cells, while memory Treg cells in the periphery as well as the naïve thymic Treg-cell pool are consistently expanded.
References
- Malek TR. The biology of interleukin-2. Annu Rev Immunol 2008; 26:453 - 79; http://dx.doi.org/10.1146/annurev.immunol.26.021607.090357; PMID: 18062768
- Josefowicz SZ, Lu LF, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol 2012; 30:531 - 64; http://dx.doi.org/10.1146/annurev.immunol.25.022106.141623; PMID: 22224781
- Beyer M, Schultze JL. Regulatory T cells: major players in the tumor microenvironment. Curr Pharm Des 2009; 15:1879 - 92; http://dx.doi.org/10.2174/138161209788453211; PMID: 19519430
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006; 107:2409 - 14; http://dx.doi.org/10.1182/blood-2005-06-2399; PMID: 16304057
- Zhang H, Chua KS, Guimond M, Kapoor V, Brown MV, Fleisher TA, et al. Lymphopenia and interleukin-2 therapy alter homeostasis of CD4+CD25+ regulatory T cells. Nat Med 2005; 11:1238 - 43; http://dx.doi.org/10.1038/nm1312; PMID: 16227988
- Sosman JA, Carrillo C, Urba WJ, Flaherty L, Atkins MB, Clark JI, et al. Three phase II cytokine working group trials of gp100 (210M) peptide plus high-dose interleukin-2 in patients with HLA-A2-positive advanced melanoma. J Clin Oncol 2008; 26:2292 - 8; http://dx.doi.org/10.1200/JCO.2007.13.3165; PMID: 18467720
- Wei S, Kryczek I, Edwards RP, Zou L, Szeliga W, Banerjee M, et al. Interleukin-2 administration alters the CD4+FOXP3+ T-cell pool and tumor trafficking in patients with ovarian carcinoma. Cancer Res 2007; 67:7487 - 94; http://dx.doi.org/10.1158/0008-5472.CAN-07-0565; PMID: 17671219
- Beyer M, Schumak B, Weihrauch MR, Andres B, Giese T, Endl E, et al. In vivo expansion of naïve CD4+ CD25(high) FOXP3+ regulatory T cells in patients with colorectal carcinoma after IL-2 administration. PLoS One 2012; 7:e30422; http://dx.doi.org/10.1371/journal.pone.0030422; PMID: 22276195
- Valmori D, Merlo A, Souleimanian NE, Hesdorffer CS, Ayyoub M. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest 2005; 115:1953 - 62; http://dx.doi.org/10.1172/JCI23963; PMID: 16007258
- Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity 2009; 30:899 - 911; http://dx.doi.org/10.1016/j.immuni.2009.03.019; PMID: 19464196