Abstract
Under physiological conditions, the trans-presentation of interleukin-15 (IL-15) by the IL-15 receptor α on the cell surface allows to confine and tune the IL-15-mediated immune responses. Therefore, targeting strategies that mimic this situation at the tumor sites appear especially promising for anticancer immunotherapy.
Interleukin (IL)-15 belongs to the common receptor γ-chain cytokine family, which also includes IL-2, IL-4, IL-7, IL-9 and IL-21. IL-15 plays an important role in the development, activity and persistence of natural killer (NK) cells and CD8+ T cells, showing great potential to promote antitumor immune responses. Thus, next to IL-2, which has been approved for treatment of metastatic renal cell carcinoma and metastatic melanoma, IL-15 is attracting increasing attention, ranking on the top of the National Cancer Institute’s list of agents considered to have high potential for cancer immunotherapy.Citation1 Although IL-15 shares many functions with IL-2, like the ability to stimulate the proliferation of NK and T cells, the generation of cytotoxic T cells and the activation of NK cells, it also has properties different from that of IL-2. Thus, unlike IL-2, IL-15 seems not to be involved in the activation-induced cell death (AICD) of effector CD8+ T cells and in the maintenance of regulatory T cells. Furthermore, preclinical studies suggest that IL-15 might be less toxic than IL-2. Hence, benefits in terms of efficacy and safety are expected, and several clinical trials involving IL-15 are currently being conducted.Citation2
IL-2 and IL-15 share the β and common γ chain of their receptors (IL-2/15Rβγc), but specifically bind to their respective α receptor chains (IL-2Rα/ IL-15Rα). IL-15 binds intracellularly with high affinity to IL-15Rα, forming a complex that shuttles from the endoplasmic reticulum to the cell surface, where IL-15Rα presents IL-15 mainly in trans to IL-2/15Rβγc expressing neighbor cells, although cis-presentation is also possible. Recycling of the whole complex between the cell surface and endosomes has been described. Thus, under physiological conditions, a confined, long-lasting membrane presentation of IL-15 can be achieved that favors a highly regulated system.Citation3
Although IL-2/15Rβγc can be stimulated by IL-15 alone, it was shown in mice that a soluble complex of IL-15 and IL-15Rα-Fc is much more effective, leading to stronger proliferation of memory CD8+ T cells and NK cells in vitro and in vivo. Also antitumor activity was increased, reducing tumor burden and increasing the survival of B16 melanoma-bearing mice.Citation4,Citation5 Similar results have been reported on a fusion protein composed of IL-15 and the fragment of the IL-15Rα chain involved in ligand binding (extended sushi domain). In comparison with IL-15 alone, treatment with the fusion protein was more efficient in mouse lung or liver metastatic melanoma (B16F10 cells) models as well in a human colon carcinoma xenograft (HCT 116 cells) model, as demonstrated by reduced metastasis formation, tumor growth inhibition and increased survival.Citation6 Thus, the presentation of IL-15 in the context of the IL-15Rα chain seems to clearly improve the antitumor potential of this cytokine.
The relevance of localized IL-15 presentation at the tumor site for anticancer immune responses was shown in a Rag1−/− mouse model bearing IL-15-secreting tumor cells. Large tumors grew in the presence of neutralizing IL-15 antibodies, but were eradicated by an NK cell-mediated immune response upon antibody withdrawal. In this setting, the co-expression of IL-15Rα by tumor cells was required for the efficient induction of densely granulated NK effector cells in the tumor microenvironment. The regression of IL-15-secreting tumors did not stop the growth of contralateral non-IL-15-secreting control tumors, suggesting that the effect of IL-15 was restricted to the local microenvironment.Citation7 In a pancreatic tumor RIP1-Tag2 mouse model, regression of established solid tumors after IL-15/IL-15Rα-Fc complex treatment could be ascribed to the stimulation of endogenous, tumor-resident CD8+ T cells.Citation8 In addition, targeted delivery of IL-15 as an EDB-directed antibody-IL-15 fusion protein resulted in the accumulation of the fusion protein in the tumor and consecutive growth inhibition in a syngeneic F9 teratocarcinoma mouse model.Citation9 Thus, the localization of IL-15 at the tumor site constitutes an important factor for the generation of efficient antitumor immune responses.
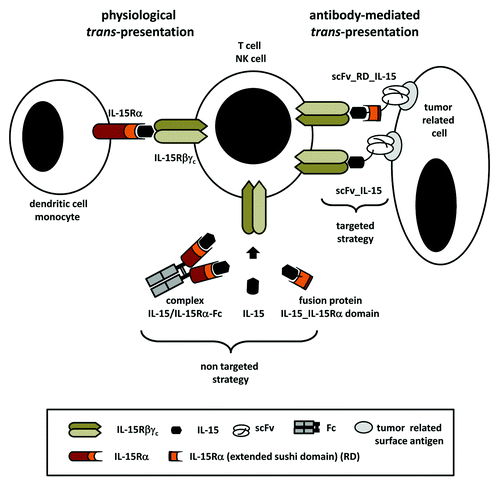
Recently, by generating a fusion protein composed of a tumor-directed antibody, IL-15 and a IL-15Rα chain domain, we have combined both aspects: presentation of IL-15 in the IL-15Rα context and targeting IL-15 presentation to tumor cells, mimicking the physiologic trans-presentation of IL-15 at the tumor site ().Citation10 We could show in vitro, that targeted presentation of IL-15 linked to the extended sushi domain of IL-15Rα (RD) was more effective in stimulating T cells in terms of proliferation and cytotoxicity than the targeted presentation of IL-15 alone. In addition, we demonstrated in a metastatic B16-transfectant tumor mouse model that optimal antitumor effects were achieved by the antibody-RD-IL-15 fusion protein, while both antibody-IL-15 or non-targeted RD-IL-15 were less effective. Thus, this particular strategy of mimicking physiological trans-presentation of IL-15 at the tumor site seems to be a promising approach for an improved application of IL-15 in cancer immunotherapy.
Figure 1. Schematic illustration of interleukin-15 trans-presentation. Under physiological conditions interleukin-15 (IL-15) binds to the IL-15Rα chain and is presented on the cell surface of dendritic cells and monocytes in trans to IL-15Rβγc expressing T or NK cells. Trans-presentation can be simulated in a non-targeted form in solution by complexing or fusing IL-15 to a ligand binding fragment of the IL-15Rα chain. Alternatively, trans-presentation can be achieved in a targeted manner by fusing IL-15 alone or in combination with the interacting IL-15Rα domain to a tumor-directed antibody moiety. Thus, antibody-mediated binding of the fusion protein mimics cell surface presentation of IL-15 at the tumor site. scFv, single-chain Fv.
Financial Support
Deutsche Forschungsgemeinschaft (MU 2956/2–1)
References
- Cheever MA. Twelve immunotherapy drugs that could cure cancers. Immunol Rev 2008; 222:357 - 68; http://dx.doi.org/10.1111/j.1600-065X.2008.00604.x; PMID: 18364014
- Steel JC, Waldmann TA, Morris JC. Interleukin-15 biology and its therapeutic implications in cancer. Trends Pharmacol Sci 2012; 33:35 - 41; http://dx.doi.org/10.1016/j.tips.2011.09.004; PMID: 22032984
- Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett 2010; 127:85 - 92; http://dx.doi.org/10.1016/j.imlet.2009.09.009; PMID: 19818367
- Stoklasek TA, Schluns KS, Lefrançois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol 2006; 177:6072 - 80; PMID: 17056533
- Dubois S, Patel HJ, Zhang M, Waldmann TA, Müller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol 2008; 180:2099 - 106; PMID: 18250415
- Bessard A, Solé V, Bouchaud G, Quéméner A, Jacques Y. High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther 2009; 8:2736 - 45; http://dx.doi.org/10.1158/1535-7163.MCT-09-0275; PMID: 19723883
- Liu RB, Engels B, Arina A, Schreiber K, Hyjek E, Schietinger A, et al. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res 2012; 72:1964 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-11-3208; PMID: 22374983
- Epardaud M, Elpek KG, Rubinstein MP, Yonekura AR, Bellemare-Pelletier A, Bronson R, et al. Interleukin-15/interleukin-15R alpha complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res 2008; 68:2972 - 83; http://dx.doi.org/10.1158/0008-5472.CAN-08-0045; PMID: 18413767
- Kaspar M, Trachsel E, Neri D. The antibody-mediated targeted delivery of interleukin-15 and GM-CSF to the tumor neovasculature inhibits tumor growth and metastasis. Cancer Res 2007; 67:4940 - 8; http://dx.doi.org/10.1158/0008-5472.CAN-07-0283; PMID: 17510424
- Kermer V, Baum V, Hornig N, Kontermann RE, Müller D. An antibody fusion protein for cancer immunotherapy mimicking IL-15 trans-presentation at the tumor site. Mol Cancer Ther 2012; In press