Abstract
In the intestine, a large variety of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) can instigate innate immune responses, which have been shown to promote colorectal carcinogenesis. We have recently demonstrated an important role for the receptor for advanced glycation end products (Rage) in intestinal adenoma formation. Rage is a receptor for DAMPs that are present in several proteins produced in intestinal adenomas. We found that Rage signaling upholds a pro-inflammatory milieu through a feed-forward loop that stimulates the production of its own ligands.
The link between cancer and the immune system has been firmly established. The influence of the immune system on tumor development is most pronounced when cancer develops in the context of chronic inflammation, such as in hepatitis-associated hepatocellular carcinoma or in colitis-associated intestinal cancer.Citation1,Citation2 However, a role for the immune system in sporadic carcinogenesis is also being increasingly recognized. During tumorigenesis, the immune system has a paradoxical role.Citation3 The adaptive immune system functions mainly as a tumor surveillance machinery, protecting tissues from malignantly transformed cells. On the contrary, the innate immune system can play a tumorigenic role, its activation being mediated by a variety of microbe-derived substances, or by factors that are released from damaged cells. These two subsets of factors that activate the innate immune system are known as pathogen- and damage-associated molecular patterns (PAMPs and DAMPs), respectively. A major signaling pathway for PAMPs proceeds via Toll-like receptors (TLRs) that are expressed on the plasma membrane of immune cells. Downstream signaling involves the activation of mitogen-activated protein kinases (MAPKs) as well as that of the transcription factor NF-κB, both of which are also implicated in tumor development. DAMPs may signal through the receptor for advanced glycation end products (Rage). Similar to TLRs, Rage is located on the plasma membrane and although its extracellular domain is distinct from the conserved region of TLRs, the effects of Rage and TLR signaling are comparable, and may even be mediated through the same adaptor molecule, MyD88.Citation4
The development of colitis-associated tumors is driven by the innate immune system, both PAMPs and DAMPs contributing to tumor formation.Citation2,Citation5 More surprisingly, innate immunity also plays a pivotal role in sporadic intestinal tumorigenesis.Citation6 In the intestine of mice in which TLR-signaling is incapacitated by the absence of the adaptor molecule MyD88, tumor development is markedly attenuated. However, the receptors or ligands that activate the innate immune system in this setting have not been identified to date.
Tumors have been described as wounds that do not heal and the tumor environment contains a high level of cellular damage signals.Citation7,Citation8 Tumor cells actively produce or passively allow for the release of DAMPs, which may signal to sustain a tumorigenic environment. We have explored the role of DAMPs that signal through Rage in a model of sporadic intestinal tumorigenesis.Citation9 We crossed mice that lack Rage due to homologous recombination (Rage−/−) to ApcMin/+ mice, harboring a truncating mutation in the adenomatous polyposis coli (Apc) gene, which is often the first gene mutated in the development of colon cancer in humans. ApcMin/+ mice that lack Rage had a 58% reduction in polyp number as compared with control animals. ApcMin/+Rage−/− animals exhibit a marked increase in tumor cell apoptosis, suggesting that their phenotype may reflect a role for Rage signaling in the survival of tumor cells. To investigate whether the levels of DAMPs are increased in tumors, we analyzed expression of a number of molecules that are known activators of Rage, including Hmgb1, S100a8i and S100a9. Comparing mRNA expression in tumors and normal tissue, Hmgb1 and S100a8, but not S100a9, were upregulated in tumors. Surprisingly, such upregulation was not observed in tumors developing in Rage-deficient animals. Finally, since skin tumor development through Rage is known to depend on immune cell infiltration,Citation10 we analyzed the effect of Rage signaling on the composition of the immune cell infiltrate in intestinal polyps. Although Rage−/− polyps did not exhibit altered infiltration of neutrophils, macrophages or T-lymfocytes, we found a surprising 14-fold upregulation in tumor-infiltrating mast cells in the adenomas from Rage−/− mice, compared with their Rage-proficient counterparts.
Our findings have implications for various aspects of sporadic tumor development in the intestine. Most importantly, DAMPs from the tumor environment activate Rage signaling and thereby contribute to tumorigenesis, perhaps by conferring survival signals to cancer cells. Second, DAMP signaling in tumors through Rage activates a self-sustaining pro-inflammatory feed-forward signaling loop. Third, in the absence of this inflammatory milieu, mast cells appear to infiltrate tumors. These cells may be involved in the protection from tumorigenesis that we observed in Rage−/− animals, although this remains speculative.
Our work identified a feed-forward pro-inflammatory loop that activates a tissue damage response through Rage signaling and plays a role in intestinal tumorigenesis. Mice that lack Rage fail to develop such a tissue damage response and they exhibit lower rates of tumor formation. Blocking DAMP signaling may thus diminish the activation of the innate immune system during tumor development and hence inhibit tumorigenesis. Thus, Rage may constitute a therapeutic target for the treatment of sporadic intestinal cancers.
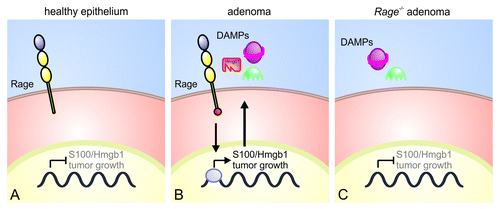
Figure 1. Rage signaling in intestinal tumorigenesis (Fig. 1). In the healthy intestinal epithelium (A), low levels of damage-associated molecular patterns (DAMPs) fail to activate Rage. In intestinal adenomas (B), high levels of DAMPs derive from damaged or dying cells. These DAMPS activate Rage, which increases the production of DAMPs such as HMGB1 and S100, which in turn sustain an inflammatory and pro-tumorigenic milieu. In the absence of Rage (C), DAMPs fail to initiate an inflammatory response, resulting in limited tumorigenesis.
References
- Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 2007; 317:121 - 4; http://dx.doi.org/10.1126/science.1140485; PMID: 17615358
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell 2004; 118:285 - 96; http://dx.doi.org/10.1016/j.cell.2004.07.013; PMID: 15294155
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer 2006; 6:24 - 37; http://dx.doi.org/10.1038/nrc1782; PMID: 16397525
- Sakaguchi M, Murata H, Yamamoto K, Ono T, Sakaguchi Y, Motoyama A, et al. TIRAP, an adaptor protein for TLR2/4, transduces a signal from RAGE phosphorylated upon ligand binding. PLoS One 2011; 6:e23132; http://dx.doi.org/10.1371/journal.pone.0023132; PMID: 21829704
- Turovskaya O, Foell D, Sinha P, Vogl T, Newlin R, Nayak J, et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 2008; 29:2035 - 43; http://dx.doi.org/10.1093/carcin/bgn188; PMID: 18689872
- Rakoff-Nahoum S, Medzhitov R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 2007; 317:124 - 7; http://dx.doi.org/10.1126/science.1140488; PMID: 17615359
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986; 315:1650 - 9; PMID: 3537791
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011; 144:646 - 74; http://dx.doi.org/10.1016/j.cell.2011.02.013; PMID: 21376230
- Heijmans J, Büller NV, Hoff E, Dihal AA, van der Poll T, van Zoelen MA, et al. Rage signalling promotes intestinal tumourigenesis. Oncogene 2012; http://dx.doi.org/10.1038/onc.2012.119; PMID: 22469986
- Gebhardt C, Riehl A, Durchdewald M, Németh J, Fürstenberger G, Müller-Decker K, et al. RAGE signaling sustains inflammation and promotes tumor development. J Exp Med 2008; 205:275 - 85; http://dx.doi.org/10.1084/jem.20070679; PMID: 18208974