Abstract
According to common beliefs, conventional anticancer chemotherapy is deleterious for the immune system. We have recently provided in vitro evidence indicating that conventional chemotherapy may potentiate, rather than impair, the long-term efficacy of γδ T cell-based anticancer immunotherapy.
Chemotherapy remains one of the most widely employed therapeutic options against malignant conditions but its efficacy is limited and, especially in the case of solid tumors, it rarely exerts a fully curative activity. Immunotherapy is emerging as an alternative approach to treat cancer patients, but it is also curative in a limited fraction of cases. So far, only a few studies have investigated approaches to combine chemotherapy and immunotherapy, mostly because these two forms of treatment have long been viewed as antagonistic strategies. In fact, as chemotherapeutic drugs often display a limited specificity, virtually all proliferating cells, including leukocytes, are susceptible to their cytotoxic effects. Thus, leukocytopenia is a common side effect of cytotoxic chemotherapy and constitutes one of the main reasons why chemotherapy and immunotherapy have long been considered as mutually, exclusive, if not antagonistic, treatment modalities.
Recent studies have challenged the assumption that chemotherapy is intrinsically detrimental for the efficacy of immunotherapy. This change in perspective may have a profound impact on cancer therapy, especially in view of the ever more precise characterization of so-called “cancer-initiating cells” (CICs), which nowadays are considered to be responsible for setting off and sustaining tumor growth.Citation1 CICs are resistant to commonly used chemotherapeutics, mainly due to (1) their location within a hypoxic niche; (2) their reduced proliferative rate; (3) an improved DNA repair capacity; and (4) the overexpression of antiapoptotic molecules.Citation2 However, conventional chemotherapy may offer an unexpected opportunity to improve the efficacy of immunotherapy. Chemotherapy enhances indeed the sensitivity of tumor cells to the cytotoxic activity of natural killer (NK) cells, γδ or CD8+ T lymphocytes. Thus, combining immunotherapy with chemotherapy may bring about significant clinical benefits to (at least a fraction of) cancer patients.Citation3
Vγ9Vδ2 T cells, the major subset of circulating γδ T cells, are good candidates for such a combinatorial approach to anticancer therapy, mainly due to their capacity to recognize target cells in a MHC-unrestricted way, to respond to phosphoantigens synthesized by the mevalonate pathway, and to exert robust antitumor effects.Citation4 Physiological levels of phosphoantigens generally fail to stimulate the immune system, but malignant cells produce increased levels of such metabolic intermediates, making them susceptible to recognition and killing by Vγ9Vδ2 T cells. Accordingly, the administration of aminobisphosphonates such as zoledronate (operating as inhibitors of farnesyl pyrophosphate synthase) to cancer cells cause the accumulation of endogenous isoprenoids, hence increasing their susceptibility to Vγ9Vδ2 T-cell cytotoxicity, which is mediated by the perforin-granzyme, CD95/CD95 ligand (CD95L), tumor necrosis factor (TNF)/TNF receptor (TNF/TNFR) and TNF-related apoptosis-inducing ligand (TRAIL)/TRAIL receptor (TRAILR) systems. Additional lines of evidence point to γδ T cells as to ideal candidates for combinatorial chemoimmunotherapy. In particular, (1) chemotherapy sensitizes differentiated malignant cell lines to the cytotoxic activity of Vγ9Vδ2 T cells;Citation5 (2) chemotherapy-induced anticancer immune responses in the mouse are strictly γδ T cell-dependent;Citation6 and (3) zoledronate makes colon CICs susceptible to Vγ9Vδ2 T-cell killing.Citation7 Taken together, these observations predict that chemotherapy and γδ T cell-based immunotherapy may exert synergistic anticancer effects.
We have recently tested this possibility in vitro by combining chemotherapy with Vγ9Vδ2 T cells to efficiently target colon CICs. In particular, since colon CICs are resistant to either of these therapeutic modalities employed as a standalone intervention, we tested whether chemotherapy sensitizes colon CICs to the cytotoxic activity of Vγ9Vδ2 T cells.Citation8
Thus, two antineoplastic agents that are largely employed in the treatment of CRC patients, namely, 5-fluorouracil (5-FU) and doxorubicin, failed to kill five different colon CIC lines over a 24–72 h treatment period, even when used at very high doses. Conversely, both 5-FU and doxorubicin exerted robust antineoplastic effects against differentiated CRC cell lines. Along similar lines Vγ9Vδ2 T cell lines obtained from CRC patients or healthy donors failed to kill colon CIC lines, even at high effector:target (E:T) ratios, while being highly cytotoxic for differentiated CRC cell lines. Unexpectedly, the pre-incubation of colon CIC lines with 5-FU or doxorubicin sensitized them to the cytotoxic activity of allogeneic and autologous Vγ9Vδ2 T cell lines, mediating additive effects at low concentrations and almost complete lysis at high doses.
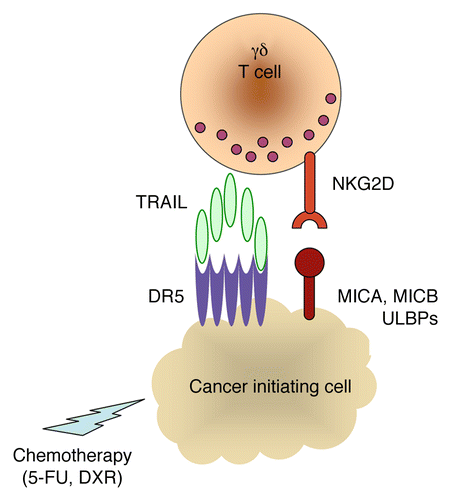
CIC lines constitutively express MHC class I molecules; intercellular adhesion molecule 1 (ICAM1); CD112; CD155; MHC class I polypeptide-related sequence (MIC)A/B; UL16-binding protein (ULBP)1–4; CD95, TNFR1 and death receptor (DR)4 (also known as TRAIL-R1), all of which were not upregulated by the administration of 5-FU and doxorubicin. Conversely, these chemotherapeutic agents significantly increased the expression levels of DR5 (TRAIL-R2) on colon CICs. Accordingly, the inhibition of the TRAIL/DR5 interaction by means of DR5-specific monoclonal antibodies limited the cytotoxic activity of Vγ9Vδ2 T cells in our model. Moreover, the killing of colon CICs by Vγ9Vδ2 T cells was significantly inhibited by natural killer group 2 member D (NKG2D)-targeting antibodies, but neither by antibodies specific for CD3 or the γδ T-cell receptor (TCR) nor by mevastatin, an inhibitor of 3-hydroxy-3-methylglutaryl-coenzyme A reductase that prevents the accumulation of endogenous phosphoantigens. This indicates that Vγ9Vδ2 T cells kill chemotherapy-sensitized colon CICs through a mechanism that involves the interaction of TRAIL with DR5 and that of NKG2D with MICA/B or ULBPs ().
Figure 1. Combinatorial antineoplastic effects of conventional chemotherapy and γδ T cell-based immunotherapy. Commonly used chemotherapeutic agents such as 5-fluorouracil (5-FU) and doxorubicin (DXR) stimulate colon cancer-initiating cells to express increased amounts of death receptor 5 (CR5), rendering them susceptible to the TNF-related apoptosis inducing ligand (TRAIL) dependent cytotoxic activity of Vγ9Vδ2 T cells, following the natural killer group 2 member D (NKG2D)-dependent recognition of stress-induced ligands. MIC, MHC class I polypeptide-related sequence; ULBP, UL16-binding protein.
Previous studies have demonstrated that chemotherapy can render tumor cells of different origin susceptible to NK or T cell-mediated killing by upregulating the expression of death receptors, including DR5.Citation9 Our study provides the first evidence of DR5 upregulation on CICs, as this effect has previously been reported only for differentiated cancer cells. We are actually investigating whether the upregulation of DR5 by chemotherapy is a general phenomenon or is restricted to the CRC setting.
The idea of combining conventional chemotherapy with immunotherapy currently stands at a pre-clinical stage of development, mainly because these two approaches have long been considered to be mutually exclusive. Our study suggests that the activation of Vγ9Vδ2 T cells in vivo or the adoptive transfer of Vγ9Vδ2 T cells activated ex vivo, along with or immediately after the administration of conventional chemotherapy may result in substantially increased therapeutic effects and hence provide consistent clinical benefits to cancer patients. Properly designed clinical studies are required to understand the actual potential of this combinatorial chemoimmunotherapeutic regimen.
| Abbreviations: | ||
| 5-FU | = | 5-fluorouracil |
| CIC | = | cancer-initiating cell |
Disclosure of Potential Conflict of Interest
No potential conflicts of interest were disclosed.
References
- Vermeulen L, Sprick MR, Kemper K, Stassi G, Medema JP. Cancer stem cells--old concepts, new insights. Cell Death Differ 2008; 15:947 - 58; http://dx.doi.org/10.1038/cdd.2008.20; PMID: 18259194
- Dean M, Fojo T, Bates S. Tumour stem cells and drug resistance. Nat Rev Cancer 2005; 5:275 - 84; http://dx.doi.org/10.1038/nrc1590; PMID: 15803154
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012; 62:309 - 35; http://dx.doi.org/10.3322/caac.20132; PMID: 22576456
- Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013; 13:88 - 100; http://dx.doi.org/10.1038/nri3384; PMID: 23348415
- Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother 2007; 56:1285 - 97; http://dx.doi.org/10.1007/s00262-007-0279-2; PMID: 17265022
- Ma Y, Aymeric L, Locher C, Mattarollo SR, Delahaye NF, Pereira P, et al. Contribution of IL-17-producing γ δ T cells to the efficacy of anticancer chemotherapy. J Exp Med 2011; 208:491 - 503; http://dx.doi.org/10.1084/jem.20100269; PMID: 21383056
- Todaro M, D’Asaro M, Caccamo N, Iovino F, Francipane MG, Meraviglia S, et al. Efficient killing of human colon cancer stem cells by gammadelta T lymphocytes. J Immunol 2009; 182:7287 - 96; http://dx.doi.org/10.4049/jimmunol.0804288; PMID: 19454726
- Todaro M, Orlando V, Cicero G, Caccamo N, Meraviglia S, Stassi G, et al. Chemotherapy Sensitizes Colon Cancer Initiating Cells to Vγ9Vδ2 T Cell-Mediated Cytotoxicity. PLoS One 2013; 8:e65145; http://dx.doi.org/10.1371/journal.pone.0065145; PMID: 23762301
- Wang S. TRAIL: a sword for killing tumors. Curr Med Chem 2010; 17:3309 - 17; http://dx.doi.org/10.2174/092986710793176285; PMID: 20712573