Abstract
The tumor microenvironment is a complex assortment of cells that includes a variety of leukocytes. The overall effect of the microenvironment is to support the growth of tumors and suppress immune responses. Immunotherapy is a highly promising form of cancer treatment, but its efficacy can be severely compromised by an immunosuppressive tumor microenvironment. Chemotherapy and radiation treatment can mediate tumor reduction through cytotoxic effects, but it is becoming increasingly clear that these forms of treatment can be used to modify the tumor microenvironment to liberate tumor antigens and decrease immunosuppression. Chemotherapy and radiotherapy can be used to modulate the tumor microenvironment to enhance immunotherapy.
Introduction
Mainstays of cancer treatment include chemotherapy and radiotherapy that are used in various regimens as first-line treatments for most malignancies. A major mechanism of tumor inhibition by chemotherapy is undoubtedly through direct toxicity to tumor cells. A range of chemical agents are used with varied mechanisms of action including their alkylating properties and their nucleoside analog properties. The use of chemotherapeutics exploits the preferential toxicity against rapidly dividing cells, such as tumor cells. Similarly, radiation can induce DNA damage in tumor cells leading to the selective elimination of malignant cells.
However, in addition to these mechanisms, chemotherapy and radiation can have a wide range of effects on tumors including modifications to the tumor microenvironment. This can lead to the induction of inflammatory cytokines and upregulation of death receptors such as Fas, which can increase antigen availability and presentation, increase the expression of major histocompatibility molecules, normalize vessels, induce danger signals and increase T cell localization.Citation1
The tumor microenvironment is composed of cancer cells in association with a variety of other cells that comprise the stroma. Stromal cells include fibroblasts and endothelial cells in addition to a variety of leukocytes, some of which can be immunosuppressive. Such immunosuppressive leukocytes include myeloid-derived suppressor cells (MDSC),Citation2 type 2 macrophages (M2)Citation3 and T regulatory cells (Treg),Citation4 which can inhibit immunity through cell contact or through the secretion of immunomodulating cytokines including transforming growth factor-β (TGF-β)
In this review, we focus on studies demonstrating the ability of chemotherapy and radiotherapy to modulate the tumor microenvironment, resulting in the enhancement of co-administered immunotherapy.
Chemotherapy to Change the Microenvironment and Enhance Immunotherapy
Although chemotherapeutic agents are generally referred to as cytotoxic, some chemotherapeutics can conserve aspects of immunity, providing opportunities to combine chemotherapy with immunotherapy. Gemcitabine is a nucleoside analog that inhibits DNA replication. One of its main side effects is neutropenia, but this can be used to advantage in the reduction of MDSC. When used in combination with cytokines or vaccine, synergistic antitumor activity can occur associated with reduction in MDSC numbers.Citation5 An increased ratio of M1 to M2 macrophages in tumor has also been observed together with increases in the antitumor activity of CD8+ T cells and NK cells.Citation6,Citation7 Immunotherapeutics aimed at stimulating antigen presenting cells (APC) can also benefit from co-administration of gemcitabine, as observed in studies when combined with an anti-CD40 agonist antibody.Citation8 Gemcitabine alone was able to increase the frequency of CD8+ T cells within tumors, which were necessary for eradication of solid tumors. Other chemotherapeutic agents including anthracyclines can also mediate recruitment and differentiation of APC to enable tumor immunity.Citation9
Oxaliplatin, a platinum-based drug, has recently been demonstrated to induce immunogenic cell deathCitation10,Citation11 to provide increased levels of tumor antigen presentable by APCCitation12 and disrupt STAT6-mediated suppression of immune responses.Citation13 Its use in combination with an inducible adenoviral IL-12 (Ad-IL-12) system was associated with a less immunosuppressive microenvironment characterized by a reduction in intratumoral MDSC and an increased ratio of CD8+ /Treg cells.Citation14 Interestingly, in contrast to studies listed above, this effect was not seen when Ad-IL-12 was combined with gemcitabine, suggesting model-specific considerations in the action of chemotherapeutics. Indeed, despite demonstrations of the ability of chemotherapy to enhance immunity, this is not always the case. Indeed, even agents widely thought of as preserving immunity can, at least in some circumstances, potentiate the immunoregulatory capacity of MDSC leading to reduced tumor immunity.Citation15
The importance of the ability of chemotherapeutics to increase antigen availability is apparent in a study using 5-aza-2'-deoxycytidine, a demethylating agent, which induced de novo expression of a cancer testis antigen, leading to enhancement of adoptive immunotherapy of mouse breast cancer tumors.Citation16
In addition to changing the cellular composition of the tumor microenvironment, chemotherapeutics can change the cytokine profile and block regulatory cell function. Paclitaxel, a mitotic inhibitor, can reduce MDSC infiltration,Citation17 but also impair Treg functionCitation18 and induce intratumoral production of macrophage chemotactic protein, which was associated with increased effectiveness of a dendritic cell vaccine against 3LL tumors in mice.Citation19
IL-12 is an immunostimulatory cytokine able to induce cytokine production, cytolytic capacity and proliferation of T cells. In the presence of an immunosuppressive tumor microenvironment, the action of IL-12 can be suboptimal. However, when IL-12 is combined with cyclophosphamide, a reduction in tumor-associated MDSC and Treg can lead to enhanced antitumor activity.Citation20,Citation21 Importantly, the dose of cyclophosphamide in these studies is relatively low, since high doses are immunosuppressive. Similarly, costimulation of T cells through OX40 alone can lead to suboptimal antitumor responses, but when combined with cyclophosphamide a profound reduction in intratumoral Tregs can lead to eradication of established tumors in mice.Citation22
Targeted therapies using small molecules that inhibit signaling pathways represent alternative drug treatments for some malignancies with less toxic profiles, and these are also able to lead to changes in the tumor microenvironment. For example, the BRAF inhibitor, vemurafenib can reduce IL-1 secretion by melanoma cells, which can lead to reduced expression of the immune inhibitory molecules PD-L1 and PD-L2 by tumor-associated fibroblasts.Citation23 Enhanced infiltration of tumors by T cells and increased recognition of melanoma by T cells has also been reported following treatment with BRAF inhibitors.Citation24,Citation25 Other targeted therapies, such as the epidermal growth factor receptor tyrosine kinase inhibitor lapatinib, can also enhance T cell activation and their infiltration into tumors.Citation26 Therefore, targeted therapies represent attractive options for combining with immunotherapies. Indeed, adoptive immunotherapy was demonstrated to be enhanced when combined with BRAF inhibition in mouse models of melanoma.Citation27 However, some molecular pathways targeted by small molecule inhibitors can be important in the survival and function of immune system cells, and some targeted therapies can be detrimental to immune responses.Citation28,Citation29 A greater understanding of the impact of these drugs on immune system components and the tumor microenvironment will enable the design of more effective combination treatments for cancer.
Thus, the tumor microenvironment can be rendered more immunogenic by choosing particular types of chemotherapeutic agent ().
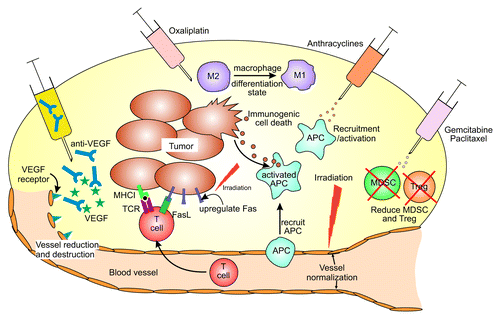
Figure 1. The effects of chemotherapy and radiotherapy on the tumor microenvironment. A range of chemotherapeutic agents can affect the tumor microenvironment in a variety of ways. Oxaliplatin can induce immunogenic cell death in a proportion of tumor cells, which can lead to the release of tumor antigens for uptake and processing by antigen presenting cells (APC). Anthracyclines can recruit APCs and enhance their differentiation to an activated phenotype, better able to present antigen to lymphocytes. Oxaliplatin can also lead to an increased proportion of proinflammatory, M1, macrophages relative to alternatively activated, M2, macrophages. Gemcitabine, oxaliplatin and paclitaxel can reduce the frequency of myeloid-derived suppressor cells (MDSC) and/or regulatory T cells (Treg) infiltrating tumors, thereby reducing their immunosuppressive effects. Tumor cells can upregulate expression of immune target molecules such as Fas and MHCI following irradiation, thereby rendering them sensitive to attack by T cells. Irradiation can also normalize dilated and chaotic blood vessels to enable T cells to access tumors. Increases in intratumoral T cells can also be achieved using antibodies against vascular endothelial growth factor (VEGF).
Radiotherapy to Enhance Immunotherapy
Several immunopotentiating events likely operate simultaneously within tumors following irradiation and, although studies rarely look at all these events and their role in the success of immunotherapy, some important connections between microenvironment changes and success of immunotherapy have been made. For example, enhanced Fas expression following localized radiotherapy was found to be important for the increased effectiveness of adoptively transferred T cells specific for CEA.Citation30 In this case, irradiation of subcutaneous mouse adenocarcinoma led to increased Fas expression and enhanced Fas-dependent CTL killing of tumor, together with a marked and significant decrease in tumor growth rate.
Similarly, two other studies demonstrated upregulation of Fas expression on tumor following localized irradiation of s.c. tumors and an enhancement of effectiveness of cancer vaccines.Citation31,Citation32 Both studies showed a dramatic influx of CD8+ cytotoxic T cells into the tumor, with associated tumor regression. Other changes were also noted including an increase in vascular density and an abscopal effect involving regression of distant unirradiated tumors.Citation32 Interestingly, induction of high levels of T cell responses against two other antigens (gp70 and p53) overexpressed in tumor was also observed (antigen cascade effect).Citation31 In the above studies, localized external beam irradiation was used, but the immunopotentiating effects have been shown to extend to other forms of radiation including brachytherapy using either 125I-seed or Yttrium-radiolabeled antibody when used in combination with vaccines.Citation33
Other immunologically important molecules upregulated by radiation include MHCI. Local tumor irradiation, demonstrated to upregulate MHCI molecules on the tumor cell surface, was combined with adoptive transfer of tumor-specific CTL to enhance the antitumor effect of transferred cells.Citation21,Citation34 In addition, novel proteins could be generated by the tumor, which were presented on the MHCI molecules and recognized by the CTL. Well-established tumors expressing low levels of antigen were treated with local irradiation, causing transient upregulation of MHC complexes on stromal cells and presentation of tumor antigen. Maximal antigen expression occurred 2 d later, and this was then combined with adoptive transfer of pre-activated CTL, causing tumor regression.
Other forms of immunotherapy besides vaccines and adoptive cell transfer can also benefit from radiotherapy. Blocking the CTLA-4 receptor to overcome T cell tolerance was used in conjunction with fractionated local irradiation (in which the total radiation dose is delivered in smaller fractions over time) to inhibit subcutaneous breast cancer tumors.Citation35 Only fractionated (and not single dose) radiotherapy worked synergistically with the anti-CTLA-4 antibody. In addition, an abscopal effect on distant tumors was observed together with a marked increase in tumor-infiltrating lymphocytes.
Different combinations of relevant monoclonal antibodies (mAbs) to stimulate immunity (anti(α)-CD137, α-CD40) and relieve immunosuppression (α-PD-1) have been combined with local irradiation in established orthotopic mammary tumors in mice.Citation36 Complete regressions were achieved using α-CD137 combined with α-PD-1 mAb and irradiation. Interestingly, in this case, single dose irradiation performed better than fractionated radiation. In these studies, treatment was associated with a temporary intratumoral enrichment of PD-1HighCD137+CD8+ T cells. Significant tumor regressions also occurred with the combination of α-CD137, α-CD40 and radiation.
It is worth noting as a final comment on the use of radiation to alter the tumor microenvironment, that radiotherapy may not always mediate positive immunopotentiating changes to the microenvironment. Indeed, in a study on glioblastoma multiforme, radiation induced recruitment of vasculogenic bone marrow-derived cells through stromal cell-derived factor-1 (SDF-1), which restored vasculature allowing tumor recurrence.Citation37
Modifying Tumor Endothelium
Irradiation and a variety of other approaches can be used to modify endothelial cells. Tumor endothelium that lines the blood vessels of tumors is composed of heterogenous cells that are organized abnormally when compared with normal blood vessel endothelium.Citation38 Tumor endothelial cells have a higher proliferative rate, the blood vessels are dilated and chaotic, with discontinuous or absent basement membrane, and abnormal pericytes cover the tumor endothelium. Researchers have targeted the tumor endothelium to correct or disrupt this abnormal endothelium development.
Ganss et al. used irradiation to cause an inflammatory response in the tumor microenvironment, involving the release of cytokines and chemokines, and upregulation of adhesion molecules.Citation39 This caused a remodeling of the tumor vasculature, due to upregulation of CXCL9 and CXCL10, enhancing vessel density in the tumors and changing their diameter so that they resembled normal capillaries. The irradiation was then followed by adoptive transfer of activated, tumor-specific lymphocytes, which previously had been unable to adhere to endothelium and access the tumor. Following irradiation, T cells were able to access and penetrate the tumor and induce complete tumor regression in some cases.
Shrimali et al. utilized another therapy, an anti-VEGF antibody that inhibits VEGF/VEGFR-2 interaction, to normalize the tumor vasculature endothelium prior to combination therapy.Citation40 Multiple doses of anti-VEGF were essential to increase extravasation into the tumor of adoptively transferred antitumor T cells following lymphodepleting conditioning. The combination therapy was required to cause reduction in tumor growth and prolonged survival of mice.
Another way to impact on tumor endothelium is to increase adhesion molecule expression on tumor endothelial vasculature. Palazon et al.Citation41 targeted CD137, which is selectively expressed on the surface of endothelial cells in response to hypoxia, with an agonist anti-CD137 monoclonal antibody. This treatment increased cell surface expression of adhesion molecules (ICAM-1, VCAM-1 and E-selectin) on tumor endothelial cells, facilitating the adhesion and extravasation of adoptively transferred lymphocytes into the tumor.
Blocking new vessel formation is another way to impact on the tumor microenvironment by increasing hypoxia and inducing apoptosis and necrosis. Manning et al.Citation42 utilized an anti-VEGF-R2 antibody, which decreased angiogenesis and increased tumor cell apoptosis. Combining this therapy with an anti-Her2 vaccine enhanced tumor regression by tumor-specific CD8+ T cells. Li et al.Citation43 also targeted VEGF receptors, but in a different way, using a recombinant adeno-associated virus vector expressing a soluble VEGF receptor. When used in combination with GM-CSF-secreting tumor cell immunotherapy a decrease in intra-tumoral Tregs, and an increase in activated CD4+ and CD8+ infiltrating effector T cells was observed, significantly enhancing the survival of the mice.
As well as endothelial cells in the tumor microenvironment, there are mesenchymal stroma cells, identified by the expression of type II membrane dipeptidylpeptidase fibroblast activation protein-α (FAP). Their suppressive function on efficacy of vaccination was ascertained in mice following FAP+ cell ablation.Citation44 Ablation of FAP+ stromal cells (which made up ~1% of all tumoral cells) combined with vaccination (VaxOVA) caused immediate tumor growth arrest with 60% decrease in viable cells in the tumor, which was dependent on TNFα and IFN-γ.
Concluding Remarks
The above review summarizes many different approaches that have been used to change the tumor microenvironment to enhance co-administered immunotherapies (). Insight gained from mouse studies into the efficacy of combining chemotherapy and/or radiotherapy with immunotherapy has been used in the design of clinical trials. Combining paclitaxel and carboplatin with anti-CD137 for the treatment of melanoma and renal cell carcinoma was well tolerated and produced some partial responses and increases in circulating CD8+ T cells.Citation45 Combining gemcitabine with agonist CD40 antibody induced partial tumor regression in 4 of 21 pancreatic ductal adenocarcinoma patients receiving the combined treatment.Citation46 Histological analysis of tumors from two patients showed regression without lymphocyte infiltrate, and a potential mechanism for regression was shown in a mouse model to be due to reeducation of tumor-associated macrophages. This study demonstrated that combination therapy in which the tumor microenvironment is modified by one or both therapies can facilitate tumor regression.
Table 1. Examples of immunotherapies that can be combined with modification of the tumor microenvironment for effective anti-tumor responses
Radiotherapy is also being used to synergize with immunotherapeutics in patients. For many years antibodies have been used to target radioisotopes to tumors, and localized modification to tumor microenvironments may well have contributed to some successes of this form of treatment.Citation47 More recently, localized radiotherapy has been used in combination with immune modulators, and increases in tumor-specific T cell frequencies demonstrated, together with some partial tumor responses.Citation48-Citation50
Immunotherapy is a highly promising treatment option for cancer, and as our understanding of the tumor microenvironment increases, we can anticipate the development of enhanced therapies utilizing immune strategies. In particular, with our developing knowledge of how chemotherapeutic agents and radiation can be used to modify the immunosuppressive nature of tumors, the full potential of immunotherapy may be able to be liberated against malignant disease.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Kershaw MH, Devaud C, John LB, Westwood JA, Darcy PK. Enhancing immunotherapy using chemotherapy and radiation to modify the tumor microenvironment. OncoImmunology 2013; 2:e25962; 10.4161/onci.25962
References
- Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol 2012; 2:104; http://dx.doi.org/10.3389/fonc.2012.00104; PMID: 22973551
- Lesokhin AM, Merghoub T, Wolchok JD. Myeloid-derived suppressor sells and the efficacy of CD8(+) T-cell immunotherapy. Oncoimmunology 2013; 2:e22764; http://dx.doi.org/10.4161/onci.22764; PMID: 23525353
- Dannenmann SR, Thielicke J, Stöckli M, Matter C, von Boehmer L, Cecconi V, Hermanns T, Hefermehl L, Schraml P, Moch H, et al. Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology 2013; 2:e23562; http://dx.doi.org/10.4161/onci.23562; PMID: 23687622
- Weiss VL, Lee TH, Jaffee EM, Armstrong TD. Targeting the right regulatory T-cell population for tumor immunotherapy. Oncoimmunology 2012; 1:1191 - 3; http://dx.doi.org/10.4161/onci.20664; PMID: 23170276
- Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res 2007; 67:7477 - 86; http://dx.doi.org/10.1158/0008-5472.CAN-06-4639; PMID: 17671218
- Fridlender ZG, Sun J, Singhal S, Kapoor V, Cheng G, Suzuki E, Albelda SM. Chemotherapy delivered after viral immunogene therapy augments antitumor efficacy via multiple immune-mediated mechanisms. Mol Ther 2010; 18:1947 - 59; http://dx.doi.org/10.1038/mt.2010.159; PMID: 20683443
- Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 2005; 11:6713 - 21; http://dx.doi.org/10.1158/1078-0432.CCR-05-0883; PMID: 16166452
- Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res 2003; 63:4490 - 6; PMID: 12907622
- Ma Y, Adjemian S, Mattarollo SR, Yamazaki T, Aymeric L, Yang H, Portela Catani JP, Hannani D, Duret H, Steegh K, et al. Anticancer chemotherapy-induced intratumoral recruitment and differentiation of antigen-presenting cells. Immunity 2013; 38:729 - 41; http://dx.doi.org/10.1016/j.immuni.2013.03.003; PMID: 23562161
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51 - 72; http://dx.doi.org/10.1146/annurev-immunol-032712-100008; PMID: 23157435
- Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2013; 2:e23510; http://dx.doi.org/10.4161/onci.23510; PMID: 23687621
- Tesniere A, Schlemmer F, Boige V, Kepp O, Martins I, Ghiringhelli F, Aymeric L, Michaud M, Apetoh L, Barault L, et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 2010; 29:482 - 91; http://dx.doi.org/10.1038/onc.2009.356; PMID: 19881547
- Lesterhuis WJ, Punt CJ, Hato SV, Eleveld-Trancikova D, Jansen BJ, Nierkens S, Schreibelt G, de Boer A, Van Herpen CM, Kaanders JH, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011; 121:3100 - 8; http://dx.doi.org/10.1172/JCI43656; PMID: 21765211
- Gonzalez-Aparicio M, Alzuguren P, Mauleon I, Medina-Echeverz J, Hervas-Stubbs S, Mancheno U, Berraondo P, Crettaz J, Gonzalez-Aseguinolaza G, Prieto J, et al. Oxaliplatin in combination with liver-specific expression of interleukin 12 reduces the immunosuppressive microenvironment of tumours and eradicates metastatic colorectal cancer in mice. Gut 2011; 60:341 - 9; http://dx.doi.org/10.1136/gut.2010.211722; PMID: 20855451
- Bruchard M, Mignot G, Derangère V, Chalmin F, Chevriaux A, Végran F, Boireau W, Simon B, Ryffel B, Connat JL, et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19:57 - 64; http://dx.doi.org/10.1038/nm.2999; PMID: 23202296
- Guo ZS, Hong JA, Irvine KR, Chen GA, Spiess PJ, Liu Y, Zeng G, Wunderlich JR, Nguyen DM, Restifo NP, et al. De novo induction of a cancer/testis antigen by 5-aza-2′-deoxycytidine augments adoptive immunotherapy in a murine tumor model. Cancer Res 2006; 66:1105 - 13; http://dx.doi.org/10.1158/0008-5472.CAN-05-3020; PMID: 16424047
- Umansky V, Sevko A. Overcoming immunosuppression in the melanoma microenvironment induced by chronic inflammation. Cancer Immunol Immunother 2012; 61:275 - 82; http://dx.doi.org/10.1007/s00262-011-1164-6; PMID: 22120757
- Zhu Y, Liu N, Xiong SD, Zheng YJ, Chu YW. CD4+Foxp3+ regulatory T-cell impairment by paclitaxel is independent of toll-like receptor 4. Scand J Immunol 2011; 73:301 - 8; http://dx.doi.org/10.1111/j.1365-3083.2011.02514.x; PMID: 21223350
- Zhong H, Han B, Tourkova IL, Lokshin A, Rosenbloom A, Shurin MR, Shurin GV. Low-dose paclitaxel prior to intratumoral dendritic cell vaccine modulates intratumoral cytokine network and lung cancer growth. Clin Cancer Res 2007; 13:5455 - 62; http://dx.doi.org/10.1158/1078-0432.CCR-07-0517; PMID: 17875775
- Medina-Echeverz J, Fioravanti J, Zabala M, Ardaiz N, Prieto J, Berraondo P. Successful colon cancer eradication after chemoimmunotherapy is associated with profound phenotypic change of intratumoral myeloid cells. J Immunol 2011; 186:807 - 15; http://dx.doi.org/10.4049/jimmunol.1001483; PMID: 21148040
- Medina-Echeverz J, Berraondo P. Colon cancer eradication after chemoimmunotherapy is associated with intratumoral emergence of proinflammatory myeloid cells. Oncoimmunology 2012; 1:118 - 20; http://dx.doi.org/10.4161/onci.1.1.18049; PMID: 22720230
- Hirschhorn-Cymerman D, Rizzuto GA, Merghoub T, Cohen AD, Avogadri F, Lesokhin AM, Weinberg AD, Wolchok JD, Houghton AN. OX40 engagement and chemotherapy combination provides potent antitumor immunity with concomitant regulatory T cell apoptosis. J Exp Med 2009; 206:1103 - 16; http://dx.doi.org/10.1084/jem.20082205; PMID: 19414558
- Khalili JS, Liu S, Rodríguez-Cruz TG, Whittington M, Wardell S, Liu C, Zhang M, Cooper ZA, Frederick DT, Li Y, et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res 2012; 18:5329 - 40; http://dx.doi.org/10.1158/1078-0432.CCR-12-1632; PMID: 22850568
- Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012; 18:1386 - 94; http://dx.doi.org/10.1158/1078-0432.CCR-11-2479; PMID: 22156613
- Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010; 70:5213 - 9; http://dx.doi.org/10.1158/0008-5472.CAN-10-0118; PMID: 20551059
- Hannesdóttir L, Tymoszuk P, Parajuli N, Wasmer MH, Philipp S, Daschil N, Datta S, Koller JB, Tripp CH, Stoitzner P, et al. Lapatinib and doxorubicin enhance the Stat1-dependent antitumor immune response. Eur J Immunol 2013; http://dx.doi.org/10.1002/eji.201242505; PMID: 23843024
- Koya RC, Mok S, Otte N, Blacketor KJ, Comin-Anduix B, Tumeh PC, Minasyan A, Graham NA, Graeber TG, Chodon T, et al. BRAF inhibitor vemurafenib improves the antitumor activity of adoptive cell immunotherapy. Cancer Res 2012; 72:3928 - 37; http://dx.doi.org/10.1158/0008-5472.CAN-11-2837; PMID: 22693252
- Rossig C. Immune modulation by molecular cancer targets and targeted therapies: Rationale for novel combination strategies. Oncoimmunology 2012; 1:358 - 60; http://dx.doi.org/10.4161/onci.18401; PMID: 22737614
- Petrelli F, Cabiddu M, Cazzaniga ME, Cremonesi M, Barni S. Targeted therapies for the treatment of breast cancer in the post-trastuzumab era. Oncologist 2008; 13:373 - 81; http://dx.doi.org/10.1634/theoncologist.2007-0173; PMID: 18448551
- Chakraborty M, Abrams SI, Camphausen K, Liu K, Scott T, Coleman CN, Hodge JW. Irradiation of tumor cells up-regulates Fas and enhances CTL lytic activity and CTL adoptive immunotherapy. J Immunol 2003; 170:6338 - 47; PMID: 12794167
- Chakraborty M, Abrams SI, Coleman CN, Camphausen K, Schlom J, Hodge JW. External beam radiation of tumors alters phenotype of tumor cells to render them susceptible to vaccine-mediated T-cell killing. Cancer Res 2004; 64:4328 - 37; http://dx.doi.org/10.1158/0008-5472.CAN-04-0073; PMID: 15205348
- Hodge JW, Sharp HJ, Gameiro SR. Abscopal regression of antigen disparate tumors by antigen cascade after systemic tumor vaccination in combination with local tumor radiation. Cancer Biother Radiopharm 2012; 27:12 - 22; http://dx.doi.org/10.1089/cbr.2012.1202; PMID: 22283603
- Chakraborty M, Gelbard A, Carrasquillo JA, Yu S, Mamede M, Paik CH, Camphausen K, Schlom J, Hodge JW. Use of radiolabeled monoclonal antibody to enhance vaccine-mediated antitumor effects. Cancer Immunol Immunother 2008; 57:1173 - 83; http://dx.doi.org/10.1007/s00262-008-0449-x; PMID: 18256832
- Zhang B, Bowerman NA, Salama JK, Schmidt H, Spiotto MT, Schietinger A, Yu P, Fu YX, Weichselbaum RR, Rowley DA, et al. Induced sensitization of tumor stroma leads to eradication of established cancer by T cells. J Exp Med 2007; 204:49 - 55; http://dx.doi.org/10.1084/jem.20062056; PMID: 17210731
- Dewan MZ, Galloway AE, Kawashima N, Dewyngaert JK, Babb JS, Formenti SC, Demaria S. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin Cancer Res 2009; 15:5379 - 88; http://dx.doi.org/10.1158/1078-0432.CCR-09-0265; PMID: 19706802
- Verbrugge I, Hagekyriakou J, Sharp LL, Galli M, West A, McLaughlin NM, Duret H, Yagita H, Johnstone RW, Smyth MJ, et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res 2012; 72:3163 - 74; http://dx.doi.org/10.1158/0008-5472.CAN-12-0210; PMID: 22570253
- Kioi M, Vogel H, Schultz G, Hoffman RM, Harsh GR, Brown JM. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest 2010; 120:694 - 705; http://dx.doi.org/10.1172/JCI40283; PMID: 20179352
- Aird WC. Endothelial cell heterogeneity. Cold Spring Harb Perspect Med 2012; 2:a006429; http://dx.doi.org/10.1101/cshperspect.a006429; PMID: 22315715
- Ganss R, Ryschich E, Klar E, Arnold B, Hämmerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res 2002; 62:1462 - 70; PMID: 11888921
- Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res 2010; 70:6171 - 80; http://dx.doi.org/10.1158/0008-5472.CAN-10-0153; PMID: 20631075
- Palazón A, Teijeira A, Martínez-Forero I, Hervás-Stubbs S, Roncal C, Peñuelas I, Dubrot J, Morales-Kastresana A, Pérez-Gracia JL, Ochoa MC, et al. Agonist anti-CD137 mAb act on tumor endothelial cells to enhance recruitment of activated T lymphocytes. Cancer Res 2011; 71:801 - 11; http://dx.doi.org/10.1158/0008-5472.CAN-10-1733; PMID: 21266358
- Manning EA, Ullman JG, Leatherman JM, Asquith JM, Hansen TR, Armstrong TD, Hicklin DJ, Jaffee EM, Emens LA. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin Cancer Res 2007; 13:3951 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-07-0374; PMID: 17606729
- Li B, Lalani AS, Harding TC, Luan B, Koprivnikar K, Huan Tu G, Prell R, VanRoey MJ, Simmons AD, Jooss K. Vascular endothelial growth factor blockade reduces intratumoral regulatory T cells and enhances the efficacy of a GM-CSF-secreting cancer immunotherapy. Clin Cancer Res 2006; 12:6808 - 16; http://dx.doi.org/10.1158/1078-0432.CCR-06-1558; PMID: 17121902
- Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010; 330:827 - 30; http://dx.doi.org/10.1126/science.1195300; PMID: 21051638
- Molckovsky A, Siu LL. First-in-class, first-in-human phase I results of targeted agents: highlights of the 2008 American society of clinical oncology meeting. J Hematol Oncol 2008; 1:20; http://dx.doi.org/10.1186/1756-8722-1-20; PMID: 18959794
- Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 2011; 331:1612 - 6; http://dx.doi.org/10.1126/science.1198443; PMID: 21436454
- Goldenberg DM, Sharkey RM. Using antibodies to target cancer therapeutics. Expert Opin Biol Ther 2012; 12:1173 - 90; http://dx.doi.org/10.1517/14712598.2012.693472; PMID: 22650606
- Chi KH, Liu SJ, Li CP, Kuo HP, Wang YS, Chao Y, Hsieh SL. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother 2005; 28:129 - 35; http://dx.doi.org/10.1097/01.cji.0000154248.74383.5e; PMID: 15725956
- Lechleider RJ, Arlen PM, Tsang KY, Steinberg SM, Yokokawa J, Cereda V, Camphausen K, Schlom J, Dahut WL, Gulley JL. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res 2008; 14:5284 - 91; http://dx.doi.org/10.1158/1078-0432.CCR-07-5162; PMID: 18698048
- Slovin SF, Higano CS, Hamid O, Tejwani S, Harzstark A, Alumkal JJ, Scher HI, Chin K, Gagnier P, McHenry MB, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol 2013; 24:1813 - 21; http://dx.doi.org/10.1093/annonc/mdt107; PMID: 23535954
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203:1259 - 71; http://dx.doi.org/10.1084/jem.20052494; PMID: 16636135