Abstract
The therapeutic success of immunotherapy requires specific alterations of the tumor microenvironment and/or the inhibition of tumor-elicited immunosuppression. Tumor-associated macrophages (TAMs) are a major component of the tumor microenvironment. We have recently shown that modulating TAMs dramatically augments the efficacy of immunotherapy. TAM-activating agents should hence be considered as an addition to immunotherapy in future clinical trials.
Although several immunotherapeutic approaches (including anticancer vaccines and adoptive T-cell transfer) have been shown to result in the accumulation of tumor-targeting cytotoxic T lymphocytes (CTLs) in the blood, the success of immunotherapy in patients with solid tumors has been limited.Citation1 This is presumably due to the robust immunosuppressive environment that is established within neoplastic lesions by both cancer and immune cells, which strongly inhibits the antineoplastic activity of cytotoxic T lymphocytes.Citation2 Thus, the generation of tumor-specific CTLs is necessary, but not sufficient, for an effective anticancer immune response.Citation2 Given this limitation, it is becoming increasingly apparent that successful immunotherapy also needs to limit tumor-induced immunosuppression, i.e., “to inhibit the inhibitors.”Citation1,Citation2 The recent clinical successes of monoclonal antibodies targeting cytotoxic T lymphocyte-associate protein 4 (CTLA4) and programmed cell death 1 (PDCD1, best known as PD-1) further illustrate this point.Citation1
Tumor-associated macrophages (TAMs) are one of the major cellular components of the tumor microenvironment, exerting a significant functional influence over it. In early-stage tumors, TAMs appear to have an inflammatory, tumoricidal (M1 or “classically activated”) phenotype. M1 macrophages exhibit a phagocytic and antigen-presenting activity, produce TH1 cytokines, and mediate cytotoxic functions. They may also promote cytotoxicity indirectly, by activating other cells of the immune system, such as natural killer (NK) and T lymphocytes.Citation3 However, as neoplastic lesions progress, macrophages polarize toward an “alternatively activated” or M2-like phenotype, differing from M1 TAMs in receptor pattern expression, antigen-presenting capacity, metabolic activity (notably arginine metabolism) and cytokine production. M2-like TAMs are thought to exert tumor-supporting, angiogenic and immunosuppressive effects,Citation3 and may contribute to the failure of immunotherapy.
TAMs thus represent a potential target for anticancer immunotherapy. Even before the concept of immunosuppressive TAM was formally popularized, investigators used lipopolysaccharide (LPS) and LPS analogs in the attempt to activate TAMs in situ. Although somewhat effective in this regard, LPS is quite toxic and elicits systemic adverse effects. A variety of other TAM-targeting therapies have been tested in preclinical models (including TAM depletion, differentiation, reprogramming, and activation), a setting in which they are associated with some degree of antineoplastic activity.Citation4
Our group has conducted studies of TAM activation using 5,6-dimethylxanthenone-4 acetic acid (DMXAA, Vadimezan), a small flavonoid-like compound originally developed as a vascular disrupting agent.Citation5,Citation6 Although endothelial cells may be directly affected by DMXAA, we and others found that this compound has additional, powerful effects on the tumor microenvironment in mouse tumor models. We showed that DMXAA administered as monotherapy is able to stimulate TAMs to secrete inflammatory cytokines and chemokines, in turn promoting endogenous CD8+ T-cell immunity and resulting in partial antitumor responses.Citation5 In contrast to previously studied TAM activators, DMXAA is highly soluble, easily administered and well tolerated. We thus used DMXAA to test the hypothesis that macrophage activation would augment the efficacy of immunotherapy.Citation6
We have recently shown that DMXAA significantly increases the efficacy of adenoviral and listeria-based anticancer vaccines against established murine tumors.Citation6 Mechanistically, we showed that DMXAA does not cause a pronounced change in the abundance of TAMs, but can shift their phenotype from M2-like to M1-like. We also observed increased amounts of tumor-infiltrating CD8+ T cells, which exhibited an improved activation status in mice receiving DMXAA plus immunotherapy as compared with mice treated only with immunotherapy. This was associated with the secretion of an immunostimulatory cytokine/chemokine cocktail in the tumor microenvironment, as assessed by RT-PCR.Citation6
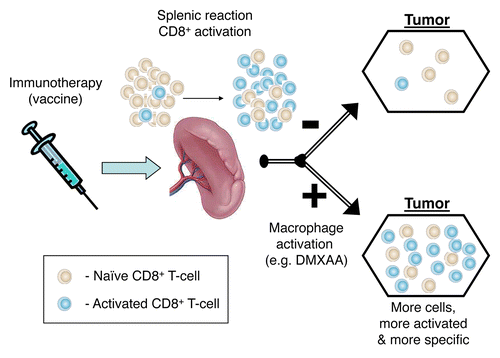
We believe that our vaccines were effective in generating antigen-specific CTLs, but that these cells trafficked poorly to neoplastic lesions and were inactivated upon tumor infiltration. The administration of DMXAA stimulated macrophage activation, resulting not only in augmented trafficking of vaccine-induced CTLs to neoplastic lesions, but also in the generation of a tumor microenvironment that did not inhibit T-cell function ().
Figure 1. Impact of tumor-associated macrophages on tumor infiltration and activation of cytotoxic T lymphocytes. Vaccination activates splenic CD8+ cytotoxic T lymphocytes (CTLs), yet only a small fraction of these cells infiltrate neoplastic lesions. Conversely, following the activation of tumor-associated macrophages (TAMs), not only an increased amount of CTLs enters the tumor, but these cells also exhibit an improved activation status and specificity.
DMXAA is not a “pure” macrophage-activating agent but also has effects on other cellular components of the tumor stroma, such as dendritic and endothelial cells.Citation5,Citation7 We believe, however, that the effect of DMXAA on TAMs is very important for 2 reasons. First, the phenotype of TAMs is altered in the course of DXMAA-based therapy. Second, macrophage depletion studies (based on clodronate-loaded liposomes) show that the loss of TAMs significantly reduces the efficacy of this immunotherapeutic approach.
It should be noted that although DMXAA potently activates murine stromal cells, it has a reduced stimulatory activity toward human cells.Citation7 We believe that this explains why 2 recent clinical trials launched by Novartis to test DMXAA in combination with chemotherapy in lung cancer patients have been discontinued due to a lack of efficacy.Citation8 This species-specificity has long remained unexplained, as decades of research failed to clarify the biochemical mechanisms by which DMXAA activates murine leukocytes. Since the publication of our manuscript, however, several groups have made a major breakthrough in this respect, showing that the intracellular target of DMXAA is a pattern recognition receptor specific for cyclic dinucleotides commonly known as stimulator of interferon genes protein (STING).Citation9,Citation10 Interestingly, DMXAA effectively binds to— hence activating—murine STING, but not the highly homologous human STING.Citation10
Our paper demonstrated that modulating the phenotype of TAMs can dramatically augment the effect of immunotherapy in murine models of lung cancer. Although it will not be possible to use DMXAA in humans, the principle of activating or depleting TAMs in combination with immunotherapy holds great therapeutic promises. It should now be possible to develop DMXAA-like drugs that activate human STING and may hence be tested as immunotherapy-boosting agents in clinical settings. As ever more effective approaches to elicit antitumor T cells and to selectively alter the tumor microenvironment are being developed, combinatorial regimens are expected to gain momentum in cancer therapy.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Fridlender ZG, Albelda SM. Modifying tumor associated macrophages: An important adjunct to Immunotherapy. OncoImmunology 2013; 2:e26620; 10.4161/onci.26620
References
- Kirkwood JM, Butterfield LH, Tarhini AA, Zarour H, Kalinski P, Ferrone S. Immunotherapy of cancer in 2012. CA Cancer J Clin 2012; 62:309 - 35; http://dx.doi.org/10.3322/caac.20132; PMID: 22576456
- Finn OJ. Cancer immunology. N Engl J Med 2008; 358:2704 - 15; http://dx.doi.org/10.1056/NEJMra072739; PMID: 18565863
- Mantovani A, Germano G, Marchesi F, Locatelli M, Biswas SK. Cancer-promoting tumor-associated macrophages: new vistas and open questions. Eur J Immunol 2011; 41:2522 - 5; http://dx.doi.org/10.1002/eji.201141894; PMID: 21952810
- Sica A, Rubino L, Mancino A, Larghi P, Porta C, Rimoldi M, Solinas G, Locati M, Allavena P, Mantovani A. Targeting tumour-associated macrophages. Expert Opin Ther Targets 2007; 11:1219 - 29; http://dx.doi.org/10.1517/14728222.11.9.1219; PMID: 17845147
- Jassar AS, Suzuki E, Kapoor V, Sun J, Silverberg MB, Cheung L, Burdick MD, Strieter RM, Ching LM, Kaiser LR, et al. Activation of tumor-associated macrophages by the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid induces an effective CD8+ T-cell-mediated antitumor immune response in murine models of lung cancer and mesothelioma. Cancer Res 2005; 65:11752 - 61; http://dx.doi.org/10.1158/0008-5472.CAN-05-1658; PMID: 16357188
- Fridlender ZG, Jassar A, Mishalian I, Wang LC, Kapoor V, Cheng G, Sun J, Singhal S, Levy L, Albelda SM. Using macrophage activation to augment immunotherapy of established tumours. Br J Cancer 2013; 108:1288 - 97; http://dx.doi.org/10.1038/bjc.2013.93; PMID: 23481183
- Henare K, Wang L, Wang LC, Thomsen L, Tijono S, Chen CJ, Winkler S, Dunbar PR, Print C, Ching LM. Dissection of stromal and cancer cell-derived signals in melanoma xenografts before and after treatment with DMXAA. Br J Cancer 2012; 106:1134 - 47; http://dx.doi.org/10.1038/bjc.2012.63; PMID: 22415295
- Lara PN Jr., Douillard JY, Nakagawa K, von Pawel J, McKeage MJ, Albert I, Losonczy G, Reck M, Heo DS, Fan X, et al. Randomized phase III placebo-controlled trial of carboplatin and paclitaxel with or without the vascular disrupting agent vadimezan (ASA404) in advanced non-small-cell lung cancer. J Clin Oncol 2011; 29:2965 - 71; http://dx.doi.org/10.1200/JCO.2011.35.0660; PMID: 21709202
- Prantner D, Perkins DJ, Lai W, Williams MS, Sharma S, Fitzgerald KA, Vogel SN. 5,6-Dimethylxanthenone-4-acetic acid (DMXAA) activates stimulator of interferon gene (STING)-dependent innate immune pathways and is regulated by mitochondrial membrane potential. J Biol Chem 2012; 287:39776 - 88; http://dx.doi.org/10.1074/jbc.M112.382986; PMID: 23027866
- Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, Rathinam VA, Monks B, Jin T, Xiao TS, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol 2013; 190:5216 - 25; http://dx.doi.org/10.4049/jimmunol.1300097; PMID: 23585680