Abstract
The specific targeting of tumor-elicited immunosuppression is a promising strategy for the treatment of cancer. We have recently demonstrated that targeting the immunosuppressive pathway mediated by CD73-derived adenosine through the blockade of A2A/A2B adenosine receptors significantly reduced the metastatic potential of CD73+ breast carcinomas and melanomas via both immunological and non-immunological mechanisms.
Targeting tumor elicited-immunosuppressive pathways has proven to be an efficient means of boosting antitumor immunity. The potential of this approach is underlined by the clinical success of monoclonal antibodies targeting cytotoxic T lymphocyte-associated protein 4 (CTLA4), programmed cell death 1 (PDCD1, best known as PD-1) and CD274 (best known as PD-L1).Citation1 One of the immunosuppressive pathways that has largely been overlooked relies on the generation of adenosine by 5′-nucleotidase, ecto (NT5E, best known as CD73). The expression of this ectoenzyme enhances tumor progression and metastatic dissemination. In line with this notion, the expression of CD73 is a poor prognostic marker among triple negative breast cancer patients.Citation2-Citation4 The activation of both A2A and A2B adenosine receptors has been shown to suppress antitumor T-cell responses within the tumor microenvironment.Citation3,Citation5,Citation6 However, the mechanisms by which CD73 promotes metastasis were less clear. Therefore, we recently set out to investigate the molecular and cellular cascades that underlie the pro-metastatic activity of CD73.Citation7
We first investigated whether ectopic CD73 expression is sufficient to increase the metastatic potential of CD73− tumors. We chose AT-3 breast carcinoma and B16F10 melanoma cells since they constitute a weakly metastatic and a highly metastatic tumor model, respectively. In both settings, CD73 expression significantly enhanced tumor metastasis. Although the major biological function of CD73 is the generation of adenosine, this ectoenzyme has been attributed with additional roles.Citation8 Therefore, we investigated whether exogenous adenosine could mimic the pro-metastatic effects of CD73. The pre-treatment of mice with 5′(N-ethylcarboxamido) adenosine (NECA), a stable analog of adenosine that operates as a pan-agonist for A1, A2A, A2B, and A3 receptors, significantly enhanced the metastatic potential of B16F10 tumor cells. To investigate which adenosine receptors would be involved in the effects of NECA, we simultaneously treated mice with NECA and selective A2A (SCH58261) or A2B (PSB-1115) antagonists. Both SCH58261 and PSB-1115 partially reversed the pro-metastatic effects of NECA. Accordingly, a selective A2A (CGS21680) or A2B (BAY60–6583) agonist was sufficient to exacerbate tumor metastasis. Although these results indicated that the activation of A2A or A2B receptors could promote the metastatic dissemination of malignant cells, they did not formally demonstrate that this pathway would underpin the increased metastatic potential of CD73+ tumors. Therefore, we investigated the ability of these antagonists to reduce the dissemination of B16F10-CD73+ and 4T1.2 cells, breast carcinoma cells that endogenously express CD73. A2A or A2B blockade significantly reduced the metastatic potential of these cancer cell lines. To confirm that the effects of SCH58261 were mediated by the A2A receptor we investigated the metastatic dissemination of B16F10-CD73+ tumor cells in A2A-deficient (Adora2a−/−) mice. These mice were significantly protected against metastases as compared with their wild-type (WT) counterparts, confirming the pro-metastatic role of host A2A receptors.
To investigate whether the blockade of A2A or A2B receptors would reduce metastasis via an immunological mechanism, we next examined whether A2A/A2B targeting was effective in immunocompromised mice lacking natural killer (NK) and T cells. We observed that NECA promotes metastasis in these mice, albeit to a lesser extent than in WT animals. Notably, the blockade of A2A receptors was no longer effective in immunocompromised mice, indicating that A2A stimulation enhances the metastatic dissemination of cancer cells as it suppress host lymphocytes. By contrast, the blockade of A2B maintained its ability to limit metastatic dissemination in the absence of NK and T cells, indicating that A2B stimulation enhances metastasis through a distinct mechanism. Since the metastatic dissemination of B16F10 cells is known to be controlled by NK cells and that the anti-metastatic effects of A2A blockade relied on host lymphocytes, we hypothesized that such anti-metastatic effects might be due to enhanced NK-cell effector functions. Indeed, we observed that the stimulation of A2A receptors suppresses the ability of NK cells to kill B16F10 or 4T1.2 cancer cells in vitro and that the activity of the A2A antagonist SCH58261 is attenuated in perforin-deficient (Prf−/−) mice. Since the phenotype of Prf1−/− mice could potentially be explained by enhanced cytotoxic functions of either NK cells or CD8+ T lymphocytes, we analyzed the phenotype of tumor-infiltrating NK cells in mice treated with A2A or A2B antagonist. Intratumoral NK cells isolated from mice treated with SCH58261, but not PSB-1115, exhibited increased expression levels of granzyme B. Taken together, these results indicate that the blockade of A2A receptors exacerbates the cytotoxic activity of NK cells in vivo.
Although our data show an unequivocal role for A2A-mediated immunosuppression in the pro-metastatic effect of CD73, this is not the only mechanism whereby CD73 stimulates tumor metastasis. Indeed, we found that CD73 expression promotes the metastatic dissemination of tumor cells in immunocompromised mice, indicating that CD73 also exerts tumor-promoting effects via non-immunological mechanisms. Notably, the autoactivation of A2B receptors on neoplastic cells has previously been shown to enhance their invasive potential by stimulating the formation of filopodia.Citation3,Citation9 Intriguingly, it has recently been shown that CD73 can promote the metastatic dissemination of tumor cells in vivo independently of its catalytic activity, indicating that the activation of adenosine receptors may not fully account for the pro-metastatic effects of CD73.Citation10
In summary, our data indicate that CD73 favors tumor metastasis by multiple mechanisms including the generation of adenosine, and activation of A2A receptors of the host (). Our data clearly suggest that the blockade of A2A or A2B represents a potential therapeutic strategy to limit metastasis. As antagonists of these receptors are already under clinical development for other indications and show good safety profiles, the translation of our findings to cancer patients appears feasible.
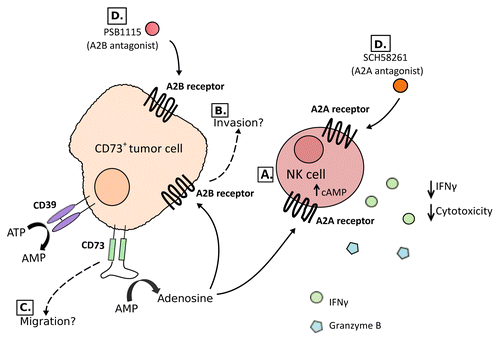
Figure 1. The expression of CD73 by malignant cells enhances metastasis through the activation of A2A and A2B adenosine receptors. (A) The expression of CD73 on malignant cells converts AMP into adenosine, which activates A2A receptors on natural killer (NK) cells. This inhibits the cytotoxic functions of NK cells as well as their ability to produce pro-inflammatory cytokines including interferon γ (IFNγ). (B) The activation of A2B receptors also stimulates metastatic dissemination through an alternative, hitherto unclear, pathway. This may potentially coincide with the activation of A2B receptors on tumor cells. (C) CD73 may also promote metastasis in an adenosine-independent manner. (D) The blockade of A2A or A2B receptors with the A2A antagonist SCH58261 or the A2B antagonist PSB-1115, respectively, significantly reduces the metastatic dissemination of CD73+ tumors.
Acknowledgements
This work was funded by a project grant from the National Health and Medical Research Council (NHMRC) and a NHMRC Senior Research Fellowship to PKD. MJS was supported by an NHMRC Australian Research Fellowship and Program Grant. JS was supported by an Operating Grant from the Canadian Institutes of Health Research.
Citation: Beavis P, Milenkovski N, Stagg J, Smyth MJ, Darcy P. A2A blockade enhances anti-metastatic immune responses. OncoImmunology 2013; 2:e26705; 10.4161/onci.26705
References
- Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013; 369:122 - 33; http://dx.doi.org/10.1056/NEJMoa1302369; PMID: 23724867
- Loi S, Pommey S, Haibe-Kains B, Beavis PA, Darcy PK, Smyth MJ, Stagg J. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A 2013; 110:11091 - 6; http://dx.doi.org/10.1073/pnas.1222251110; PMID: 23776241
- Stagg J, Divisekera U, McLaughlin N, Sharkey J, Pommey S, Denoyer D, Dwyer KM, Smyth MJ. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A 2010; 107:1547 - 52; http://dx.doi.org/10.1073/pnas.0908801107; PMID: 20080644
- Leth-Larsen R, Lund R, Hansen HV, Laenkholm AV, Tarin D, Jensen ON, Ditzel HJ. Metastasis-related plasma membrane proteins of human breast cancer cells identified by comparative quantitative mass spectrometry. Mol Cell Proteomics 2009; 8:1436 - 49; http://dx.doi.org/10.1074/mcp.M800061-MCP200; PMID: 19321434
- Ohta A, Gorelik E, Prasad SJ, Ronchese F, Lukashev D, Wong MK, Huang X, Caldwell S, Liu K, Smith P, et al. A2A adenosine receptor protects tumors from antitumor T cells. Proc Natl Acad Sci U S A 2006; 103:13132 - 7; http://dx.doi.org/10.1073/pnas.0605251103; PMID: 16916931
- Cekic C, Sag D, Li Y, Theodorescu D, Strieter RM, Linden J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol 2012; 188:198 - 205; http://dx.doi.org/10.4049/jimmunol.1101845; PMID: 22116822
- Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, Dwyer K, Stagg J, Smyth MJ, Darcy PK. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A 2013; 110:14711 - 6; http://dx.doi.org/10.1073/pnas.1308209110; PMID: 23964122
- Airas L, Niemelä J, Jalkanen S. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function-associated antigen-1-dependent mechanism. J Immunol 2000; 165:5411 - 7; PMID: 11067892
- Desmet CJ, Gallenne T, Prieur A, Reyal F, Visser NL, Wittner BS, Smit MA, Geiger TR, Laoukili J, Iskit S, et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc Natl Acad Sci U S A 2013; 110:5139 - 44; http://dx.doi.org/10.1073/pnas.1222085110; PMID: 23483055
- Terp MG, Olesen KA, Arnspang EC, Lund RR, Lagerholm BC, Ditzel HJ, Leth-Larsen R. Anti-Human CD73 Monoclonal Antibody Inhibits Metastasis Formation in Human Breast Cancer by Inducing Clustering and Internalization of CD73 Expressed on the Surface of Cancer Cells. J Immunol 2013; 191:4165 - 73; http://dx.doi.org/10.4049/jimmunol.1301274; PMID: 24043904