Abstract
We have recently defined “immunogenic modulation,” a mechanism whereby malignant cells that survive anticancer therapy, due to sublethal delivery or development of treatment resistance, become nonetheless more sensitive to killing by cytotoxic T lymphocytes. This mechanism can be exploited to identify which therapies will best synergize with immunotherapy, potentially maximizing patient clinical benefit.
Introduction
Anticancer therapies aim at eradicating malignant lesions through the direct killing of neoplastic cells. However, achieving a complete and sustained clinical remission is elusive for most patients receiving standard-of-care therapeutic regimens. Striking clinical observations in recent years indicated that patients harboring certain malignancies achieved higher clinical benefit with immunotherapy if previously treated with certain anticancer therapies. These observations are now supported by findings demonstrating that some conventional chemotherapeutic as well as emerging anticancer agents modulate the tumor to become an immunostimulatory milieu. The immunological consequences of anticancer therapy stem from the modulation of the immune system as well as from direct effects on malignant cells.Citation1 Anticancer therapies can induce a continuum of immunogenic alterations in malignant cells that can manifest in dying neoplastic cells, in the surviving cancer cell population, or on both (). These changes can be harnessed to improve the clinical benefits of subsequent or concomitant immunotherapy.
Figure 1. Rationale behind immunogenic modulation. Multiple immunogenic effects elicited by radiation therapy, conventional chemotherapeutics, small molecule inhibitors, and androgen deprivation can be exploited to improve the efficacy of anticancer immunotherapy.
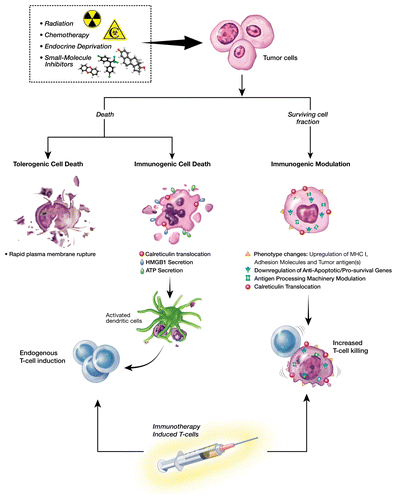
Elegant studies by the Zitvogel and Kroemer laboratories have established that malignant cells dying upon exposure to radiation or selected chemotherapeutics can elicit strong antitumor immune responses. Such a cell death modality has been designated “immunogenic cell death” (ICD).Citation2 We have examined the immunological consequences of multiple anticancer treatment modalities on the population of malignant cells that survive therapy, either because of sublethal delivery or upon the development of treatment resistance, and demonstrated the importance of combining conventional treatments with therapeutic anticancer vaccines and other immunotherapeutic platforms.Citation3-Citation7
Molecular Mechanisms of Immunogenic Modulation
Our investigations have highlighted a novel mechanism, complementary to ICD, whereby anticancer therapies alter the biology of surviving cancer cells to render them more sensitive to antigen-specific CD8+ T cell-mediated killing.Citation3-Citation7 We defined this mechanism “immunogenic modulation” (IM). IM encompasses a spectrum of molecular alterations in the biology of cancer cells that, independently or collectively, render neoplastic cells more susceptible to cytotoxic T lymphocyte (CTL)-mediated destruction. These include (1) changes in the surface phenotype of cancer cells, including the exposure of calreticulin on the outer leaflet of the plasma membrane,Citation3-Citation6 (2) the downregulation of anti-apoptotic and/or pro-survival genes,Citation1,Citation3 and (3) the modulation of components of the antigen-processing machinery (APM)Citation6,Citation7 ().
Various treatment modalities have been shown to induce IM in tumors of diverse origin by increasing the levels of immunologically relevant proteins on the surface of cancer cells, including multiple tumor-associated antigens (TAAs), adhesion molecules, e.g., intercellular adhesion molecule 1 (ICAM1), heat-shock 70 kDa protein (HSP70), and MHC class I molecules.Citation3-Citation5 These phenotypic changes, as triggered by exposure to sublethal doses of irradiation, chemotherapy with cisplatin plus vinorelbine, or androgen deprivation with enzalutamide, have been shown to translate into an increased sensitivity of murine and/or human cancer cells to CTL-mediated lysis in vitro. In a murine colon carcinoma model, the use of radiofrequency ablation (RFA) confirmed and expanded the spectrum of phenotypic changes induced by this treatment modality in vivo, resulting in sequential T-cell responses to multiple TAAs.Citation4 Importantly, combining RFA with a therapeutic vaccine targeting carcinoembryonic antigen (CEA) resulted in synergistic and effective antitumor immunity.Citation4
Enzalutamide, a second generation androgen receptor antagonist, has recently been approved by the US Food and Drug Administration (FDA) for the treatment of castration-resistant prostate carcinoma (CRPC). Enzalutamide has been shown to modulate the phenotype of murine prostate carcinoma cells, augmenting their sensitivity to CTL-mediated lysis.Citation5 Notably, the combination of enzalutamide with a therapeutic anticancer vaccine encoding twist family bHLH transcription factor 1 (TWIST1), a transcription factor involved in the metastatic process, translated into a significant increase in the overall survival (27.5 vs. 10.3 weeks) of mice bearing spontaneous prostate adenocarcinomas (TRAMP model).Citation5
Accumulating evidence suggests that the downregulation of anti-apoptotic and/or pro-survival genes may be an important component in the molecular signature of IM. A robust downregulation of multiple pro-survival genes, including anti-apoptotic members of the Bcl-2 gene family, has been observed in murine and/or human carcinoma cells exposed to sublethal doses of chemotherapy (cisplatin plus vinorelbine), chemoradiation (5-fluorouracil, cisplatin and radiation), and a small molecule BCL-2 inhibitor.Citation1,Citation3
Successful IM and the consequent CTL-mediated lysis of cancer cells is critically dependent upon the proper presentation of CD8-restricted peptide/MHC class I complexes on the surface of tumor cells, a process that is controlled by the cooperative action of multiple components of the APM. Defects in the APM of cancer cells limit T-cell recognition and correlate with poor clinical prognosis.Citation8 Recent investigations have uncovered a potential role of anticancer therapy in modulating the activity of the APM. Malignant cells recovering from radiation therapy exhibit increased sensitivity to CTL-mediated killing while displaying an altered antigenic repertoire, and show increased transporter 1, ATP-binding cassette, sub-family B (TAP1) activity.Citation1,Citation7 We have recently demonstrated that docetaxel induces IM by upregulating the expression of various APM components, in vitro and in vivo.Citation6 Furthermore, the exposure of human carcinoma cells to docetaxel, while not eliciting ICD, increased the sensitivity of surviving cancer cells to the cytotoxic activity of CTLs, an effect that was largely mediated by the translocation of calreticulin to the cancer cell surface. This process highlights a novel role for this component of the APM with potential implications for immunotherapy.Citation6
Clinical Implications
The preclinical findings described above have translated into various clinical trials, some of which have already generated encouraging preliminary data. In a randomized Phase II study, metastatic breast cancer patients who received docetaxel combined with the therapeutic anticancer vaccine PANVAC (a poxvirus-based vaccine encoding 2 TAAs, namely, CEA and mucin 1, along with a TRIad of COstimulatory Molecules, designated TRICOM) attained a superior progression-free survival (PFS) as compared with individuals receiving docetaxel alone (6.6 vs. 3.8 mo).Citation9 In an ongoing multi-center Phase II clinical trial in which metastatic CRPC patients were randomized to receive 153Sm-EDTMP (Quadramet®, from Cytogen Corp, Princeton, NJ, an FDA-approved radiopharmaceutical targeting bone metastases) alone or in combination with PSA-TRICOM, an interim analysis demonstrates improved PFS among patients receiving the combination regimens as compared with subjects treated with 153Sm-EDTMP alone, warranting continued accrual.Citation10 Thus, one can envision that the immunogenic consequences of anticancer therapy, ranging from ICD to IM, can be harnessed to maximize the clinical benefits provided by cancer vaccines and other immunotherapeutic regimens.
Disclosure of Potential Conflicts of Interest
This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, NIH. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Citation: Hodge JW, Kwilas A, Ardiani A, Gameiro SR. Attacking malignant cells that survive therapy: Exploiting immunogenic modulation. OncoImmunology 2013; 2:e26937; 10.4161/onci.26937
References
- Hodge JW, Ardiani A, Farsaci B, Kwilas AR, Gameiro SR. The tipping point for combination therapy: cancer vaccines with radiation, chemotherapy, or targeted small molecule inhibitors. Semin Oncol 2012; 39:323 - 39; http://dx.doi.org/10.1053/j.seminoncol.2012.02.006; PMID: 22595055
- Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013; 31:51 - 72; http://dx.doi.org/10.1146/annurev-immunol-032712-100008; PMID: 23157435
- Gameiro SR, Caballero JA, Higgins JP, Apelian D, Hodge JW. Exploitation of differential homeostatic proliferation of T-cell subsets following chemotherapy to enhance the efficacy of vaccine-mediated antitumor responses. Cancer Immunol Immunother 2011; 60:1227 - 42; http://dx.doi.org/10.1007/s00262-011-1020-8; PMID: 21544650
- Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, Guha C, Hodge JW. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One 2013; 8:e70417; http://dx.doi.org/10.1371/journal.pone.0070417; PMID: 23894654
- Ardiani A, Farsaci B, Rogers CJ, Protter AA, Guo Z, King TH, Apelian D, Hodge JW. Combination therapy with a second-generation androgen receptor antagonist and a aetastasis vaccine improves survival in a spontaneous prostate cancer model. Clin Cancer Res 2013; Forthcoming http://dx.doi.org/10.1158/1078-0432.CCR-13-1026; PMID: 24048332
- Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, Gameiro SR. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer 2013; 133:624 - 36; http://dx.doi.org/10.1002/ijc.28070; PMID: 23364915
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203:1259 - 71; http://dx.doi.org/10.1084/jem.20052494; PMID: 16636135
- Bangia N, Ferrone S. Antigen presentation machinery (APM) modulation and soluble HLA molecules in the tumor microenvironment: do they provide tumor cells with escape mechanisms from recognition by cytotoxic T lymphocytes?. Immunol Invest 2006; 35:485 - 503; http://dx.doi.org/10.1080/08820130600808246; PMID: 16916763
- C.R. Heery NKI, Mohebtash M, Madan R.A, Arlen P.M, Bilusic M, Kim J.W, Singh N.K, Hodge S, McMahon S, Steinberg S.M, Hodge J.W, Schlom J, Gulley J. A phase 2 randomized trial of docetaxel alone or in combination with therapeutic cancer vaccine, CEA-, MUC-1-TRICOM. Cancer research 2012; 72(24 Suppl):Abstract nr P5-16 - 06
- Heery CR, Madan RA, Bilusic M, Kim JW, Singh NK, et al. Interim analysis of a phase II randomized clinical trial of samarium-153 (Sm-153) with or without PSA-TRICOM vaccine in metastatic castration-resistant prostate cancer after docetaxel. In: American Society of Clinical Oncology: 2012; Chicago, Illinois: ASCO; 2012: 2526.