Abstract
Rapalogs such as rapamycin (sirolimus), everolimus, temserolimus, and deforolimus are indicated for the treatment of some malignancies. Rapamycin is the most effective cancer-preventive agent currently known, at least in mice, dramatically delaying carcinogenesis in both normal and cancer-prone murine strains. In addition, rapamycin and everolimus decrease the risk of cancer in patients receiving these drugs in the context of immunosuppressive regimens. In general, the main concern about the use of immunosuppressants in humans is an increased risk of cancer. Given that rapalogs are useful in cancer prevention and therapy, should they be viewed as immunosuppressants or immunostimulators? Or should we reconsider the role of immunity in cancer altogether? In addition to its anti-viral, anti-inflammatory, anti-angiogenic and anti-proliferative effects, rapamycin operates as a gerosuppressant, meaning that it inhibits the cellular conversion to a senescent state (the so-called geroconversion), a fundamental process involved in aging and age-related pathologies including cancer.
Introduction: The Paradox of mTOR Inhibitors
Rapamycin (sirolimus or Rapamune®) and its analogs such as everolimus, temsirolimus (a prodrug of rapamycin) and deforolimus inhibit mechanistic target of rapamycin (MTOR, best known as mammalian target of rapamycin, mTOR). Rapalogs have only one target, the so-called mTOR complex 1 (mTORC1),Citation1-Citation5 and exert therefore almost identical effects, including an indirect activity on mTORC2 in some models.Citation6,Citation7 Rapalogs are currently employed in several clinical applications, including cancer therapy and the management of organ transplantation. Actually, these 2 indications appear at odds with each other. The use of rapalogs in organ recipients was indeed justified by their immunosuppressive activity. As such, rapalogs were expected to increase the incidence of cancer and/or to favor tumor progression. Nonetheless, rapalogs are increasingly more used in cancer therapy.Citation8-Citation18 As a matter of fact, rapamycin and everolimus not only do not increase cancer incidence in transplant recipients but limit oncogenesis in such patients,Citation19 as we will discuss later. In other words, rapamycin and other rapalogs, which had been initially developed as immunosuppressants, turned out to be cancer-preventive agents. One possible explanation for such a dual activity of rapalogs is that mTOR is frequently hyperactivated in malignant cells.Citation15-Citation20 But this is only a part of the answer. A second explanation is indeed that rapamycin may prevent cancer by slowing down aging, being cancer an age-associated disease.Citation21-Citation23
Cancer is an Age-Related Disease
The incidence of common cancers including breast, lung, prostate, colorectal, gastric, thyroid, pancreatic, and ovarian carcinomas as well as of some types of leukemia is increased exponentially with age.Citation21,Citation22 Furthermore, cancer is a leading cause of death “from aging” in most mammals. Thus, any nutritional, pharmacological, or genetic intervention that decelerates aging (for example, caloric restriction) also postpones cancer.Citation24-Citation29 This predicts that drugs that slow down the aging process delay or prevent oncogenesis.Citation21 Do such drugs exist? And what are their targets?
mTOR and Geroconversion
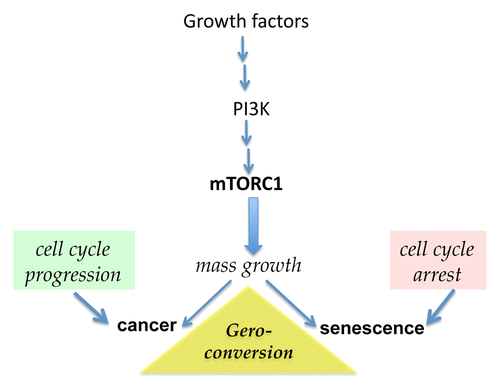
Cellular models of accelerated senescence have been useful to define the molecular and cellular bases of aging.Citation30,Citation31 Cultured cells are generally overstimulated by growth factors, nutrient-rich conditions and elevated oxygen levels. In such conditions, growth-promoting pathways such those mediated by mitogen-activated protein kinases (MAPKs) and the phosphoinositide-3-kinase (PI3K)/mTOR axis are activated. Cells grow therefore in size and then divide. Conversely, various stress conditions can cause an arrest of the cell cycle by promoting the activation of the oncosuppressor p53 and/or the accumulation of cell cycle inhibitors such as cyclin-dependent kinase inhibitor 1A (CDKN1A, best known as p21CIP1) and cyclin-dependent kinase inhibitor 2A (CDKN2A, best known as p16INK4A). When the cell cycle is arrested, cultured cells are still stimulated by growth factors and, in the case of malignant cells, oncogenic signaling pathways. All these factors activate mTOR and MAPK signaling.Citation32-Citation38 In proliferating cells, mTOR stimulates growth, replication and other cellular functions, for instance, protein synthesis. Conversely, in cells undergoing a cell cycle arrest or in quiescent cells, mTOR promotes not only hypertrophy, an hypersecretory phenotype and the hyperactivation of other cellular functions, but also signal resistance, all of which are hallmarks of senescence.Citation39-Citation42 In these conditions, cells can enter the S phase of the cell cycle but cannot divide, even when they are released from the cell cycle-arresting conditions.Citation43 Thus, an hypermitogenic drive and the unscheduled entry in the S phase of the cell cycle co-exist with the loss of proliferative and regenerative potential.Citation43,Citation44 In cells undergoing a cell cycle arrest, mTOR-dependent and mTOR-related signaling pathways drive a conversion to senescence (). In culture, a geroconversion usually takes several days, eventually resulting in the permanent acquisition by the cell of a senescent phenotype.Citation45 Rapamycin and other inhibitors of mTOR decelerate or suppress geroconversion.Citation45-Citation49 By activating mTOR, the products of multiple oncogenes accelerate geroconversion. In contrast, tumor suppressors, including phosphatase and tensin homolog (PTEN) as well as p53, decelerate geroconversion by inhibiting mTOR.Citation31,Citation50-Citation56 Therefore, p53 plays a dual role in senescence: it promotes a cell cycle arrest in response to stress but suppresses geroconversion.Citation57-Citation62 Like other oncosuppressors, p53 may exert anti-aging effects.Citation63-Citation66 Of note, mTOR is also involved in the geroconversion and exhaustion of quiescent and stem cells in the organism.Citation67,Citation68 If the mTOR-driven geroconversion constituted a cellular basis of organismal aging, the inhibition of mTOR-dependent signaling pathways would extend lifespan in animals.
Figure 1. The mTOR pathway is involved in both cancer and senescence. Growth factors, cytokines, insulin, nutrients, and oncoproteins activate the phosphoinositide-3-kinase/mammalian target of rapamycin (PI3K/mTOR) signaling pathway, which promotes cellular growth and cell cycle progression. Mutations that results in constitutive activation of the mTOR pathway are involved in oncogenic transformation when intrinsic controls on cell cycle progression are disabled. Conversely, when mTOR is activated and the cell cycle is arrested, cells enter a senescent state. Rapamycin and other rapalogs (e.g., everolimus) inhibit the mTOR complex 1 (mTORC1) and suppress such a geroconversion.
TOR Pathways is Involved in Aging
The inhibition of TOR slows aging and prolongs lifespan in diverse species.Citation69-Citation76 Importantly, rapamycin extends the lifespan of mice.Citation77-Citation82 Along similar lines, both metformin and caloric restriction, 2 interventions that inhibit mTOR signaling, also prolong the lifespan and postpone oncogenesis in mammals.Citation83-Citation86 Thus, mTOR is involved in age-related diseases and rapamycin prevents many age-related diseases.Citation87-Citation98 Do rapalogs prevent cancer in humans?
Rapalogs Decrease the Risk of Developing Cancer in Humans
Organ transplant recipients exhibit an increased incidence of lymphomas, Kaposi’s sarcomas as well as of cutaneous and hepatic cancers, at least in part due to the immunosuppression caused by corticosteroids, cyclosporine, azathioprine, and tacrolimus. Of, note, tacrolimus, an inhibitor of calcineurin, should not be confused with rapamycin (sirolimus), as it does not inhibit mTOR. In 1999, the US Food and Drug Administration approved rapamycin for the treatment of organ transplant recipients. It was expected that rapamycin would increase the risk of these individuals to develop cancer. Unexpectedly, rapamycin turned out to limit the incidence of cancers, including lymphoma, among organ transplant recipients.Citation19,Citation99-Citation103 Actually, rapamycin also cured pre-existing tumors,Citation104-Citation109 especially cutaneous Kaposi's sarcoma in kidney-transplant recipients.Citation108 In 2010, US the FDA approved everolimus for the prevention of organ rejection. Like rapamycin, everolimus also appears to limit oncogenesis among organ recipients. Thus, rapalog-containing therapeutic regimens decrease the risk of transplanted patients to develop a skin cancer.Citation110-Citation112 In line with this notion, switching from calcineurin inhibitors to rapamycin had an antitumor effect in kidney transplant recipients, decreasing the incidence of secondary squamous cell carcinomas. In particular, the number of such carcinomas developing in organ recipients treated with rapamycin was 3.4-fold lower than that arising in control patients (receiving calcineurin inhibitors).110 Thus, in several studies, the use of rapalogs has been associated with a significant decrease in cancer incidence. Only in a few studies rapalogs have been reported to exert statistically insignificant anticancer effects, in particular among patients bearing multiple pre-existing tumors.Citation110 This is consistent with an indirect cancer-preventive activity of rapalogs rather than with direct anticancer effects.Citation21,Citation22 It is important to emphasize that no clinical trial ever attributed to rapalogs a cancer-promoting activity. Thus, the warning that rapalogs might increase cancer incidence is not supported by clinical data.
It should also be noted that clinical trials recruiting transplant organ recipients aimed at comparing rapalog-containing regimens with other immunosuppressive treatment modalities. In such trials, rapamycin or everolimus were given to patients in substitution of immunosuppressants. Thus, it remains to be clearly determined whether rapalogs themselves prevent cancer or it is the withdrawal of conventional immunosuppressants such as cyclosporine and tacrolimus that plays the most critical role cancer-preventive function in this setting. To answer this question rapalogs must be directly compared with placebo or no treatment, an experimental setting that can be easily investigated in mice.
Rapamycin Prevents Cancer in Mice
Rapamycin is extremely effective in the prevention of cancer in animal models.Citation80,Citation113-Citation127 For example, when initiated 1 week after the administration of tobacco-specific carcinogens, rapamycin decreased tumor multiplicity by 90%, or 10-fold.Citation115 Along similar lines, rapamycin has been shown to increase the maximum lifespan of cancer-prone mice while slowing down the aging process.Citation120 In p53-deficient mice, which are prone to develop several tumors as they age, rapamycin extended lifespan by more than 30%.Citation118 As mentioned above, the cancer-preventive effects of rapamycin may be due to its anti-aging activity.Citation21,Citation22,Citation128
Rapalogs as Anticancer Drugs
Rapalogs are increasingly indicated for cancer therapy, in particular for the treatment of mTOR-dependent cancer subtypes.Citation9-Citation18,Citation129-Citation132 These selective mTORC1 inhibitors are preferentially effective in the context of combinatorial regimens.Citation133-Citation138 Rapalogs significantly delay cancer progression or prevent cancer relapse in breast cancer patients.Citation139,Citation140 In addition to targeting cancer cells in a direct fashion, rapalogs exert multiple indirect anticancer effects.Citation141 For instance, rapamycin inhibits the senescence of stromal cells, which is known to support tumor progression.Citation142-Citation145 Another promising application of rapamycin is the prevention of the side effects of chemotherapy, part of which originates from the senescence of normal cells (which rapamycin prevents).Citation141-Citation149 Also, hyperinsulinemia, obesity and cancer are linked through mTOR-regulated processes, suggesting that rapamycin may also be useful for the treatment of cancer-associated metabolic disorders.Citation150,Citation151
Does Immunosuppression Play Role in the Cancer-Preventive Activity of Rapalogs?
Immunosuppressive and anti-inflammatory effects are overlapping, and inflammation is well known to fosters both cancer and aging.Citation121,Citation152-Citation155 Rapalogs could thus be viewed as anti-inflammatory agents, and—at least in theory—one of the mechanisms whereby rapamycin exerts anticancer effects could be its anti-inflammatory activity.
Immunostimulation by Rapalogs
Rapamycin can improve immune responses, especially in old animals.Citation67,Citation156-Citation160 Moreover, rapamycin enhances the resistance of aged mice to pneumococcal pneumonia via a mechanism that impinges on the inhibition of cellular senescence.Citation161 Thus, rapamycin exerts both immunosuppressive and immunostimulatory organismal effects. Noteworthy, immunosurveillance systems are set in place to eliminate senescent (pre-malignant) tetraploid cancer cells.Citation162-Citation165 Therefore, tetraploidy-inducing chemotherapeutic agents (such as paclitaxel) may elicit anticancer responses by re-activating such an immunosurveillance system.Citation162,Citation163,Citation166 Rapalogs and other inhibitors of mTOR signaling may induce autophagy, which is involved in both cancer and aging.Citation167-Citation170 Autophagy is expected to boost anticancer immune responses.Citation171-Citation180Rapamycin also exerts antiviral effects in humans.Citation181,Citation182 In particular, rapamycin has been shown to reduce the risk of cytomegalovirus (CMV) infection,Citation183-Citation185 and to prevent the progression of liver fibrosis caused by the hepatitis C virus.Citation186 Moreover, rapamycin appears to control, at least in part, HIV-1 and hepatitis C virus replication. Infections from herpes zoster, herpes simplex, and human papillomavirus are common among kidney transplant recipients that are not treated with rapamycin. Along these lines, the shift from calcineurin inhibitors to rapamycin led to a relief from viral cutaneous warts among transplant recipients.Citation187 Moreover, rapamycin is effective against the Epstein-Barr virus (EBV),Citation188 it inhibits the proliferation of EBV+ B-cell lymphomasCitation189 as well as of EBV+ smooth muscle tumors.Citation190 Since viruses are involved in at least some types of cancer,Citation191-Citation193 the antiviral activities of rapalogs can contribute to their cancer-preventive effects.
Gerosuppression and Oncosuppression
Aging is the main risk factor for developing cancer and hence anti-aging drugs should exert a cancer-preventive activity. Aging is associated with metabolic, systemic, and microenvironmental changes that promote oncogenesis, a condition that can be named oncophilia. From a cellular perspective, both the oncogenic transformation and the geroconversion involve the activation of the mTOR signaling pathway, which is almost obligatory for tumorigenesis and cell senescence. One of the main differences between malignant and senescent cells is the status of the cell cycle. In cancer, the control of the cell cycle is disabled. According to this perspective, a cancer cell can be seen as a proliferating senescent cell.Citation31,Citation50 Senescent normal cells simultaneously manifest a proliferative drive (active mTOR and MAPK signaling, overexpression of cyclin D and E) and a loss of proliferative potential.Citation43,Citation44 Thus, the senescence of normal cells create a selective advantage for cells that lacking a control on their cycle.Citation194-Citation198 In this context, geroconversion shifts to oncogenic transformation.
Conclusions
Rapamycin and its analogs decrease the risk of organ transplant patients to develop cancer. Rapalogs also prolong the disease-free and overall survival of patients affected by some tumors. Furthermore, rapamycin markedly prevents oncogenesis in both normal and cancer-prone mice. Some important questions remain to be addressed. As we have reviewed here, rapamycin and everolimus decrease cancer incidence among transplant recipients as compared with other treatment modalities (such as calcineurin inhibitors) that by themselves can increase the risk of these individuals of developing tumors. Do rapalogs decrease cancer incidence as compared with placebo or no treatment? Would they decrease the incidence of cancer among immunocompetent individuals? Would they prevent cancer in healthy individuals? Finally, what should be the doses and administration modalities for rapamycin and other rapalogs to prevent cancer without causing side effects. Further studies are required to address these incognita.
Citation: Blagosklonny MV. Immunosuppressants in cancer prevention and therapy. OncoImmunology 2013; 2:e26961; 10.4161/onci.26961
References
- Hall MN. Talks about TORCs: recent advancesin target of rapamycin signalling. On mTOR nomenclature. Biochem Soc Trans 2013; 41:887 - 8; http://dx.doi.org/10.1042/BST20130092; PMID: 23863150
- Loewith R, Jacinto E, Wullschleger S, Lorberg A, Crespo JL, Bonenfant D, Oppliger W, Jenoe P, Hall MN. Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol Cell 2002; 10:457 - 68; http://dx.doi.org/10.1016/S1097-2765(02)00636-6; PMID: 12408816
- Benjamin D, Colombi M, Moroni C, Hall MN. Rapamycin passes the torch: a new generation of mTOR inhibitors. Nat Rev Drug Discov 2011; 10:868 - 80; http://dx.doi.org/10.1038/nrd3531; PMID: 22037041
- Kang SA, Pacold ME, Cervantes CL, Lim D, Lou HJ, Ottina K, Gray NS, Turk BE, Yaffe MB, Sabatini DM. mTORC1 phosphorylation sites encode their sensitivity to starvation and rapamycin. Science 2013; 341:1236566; http://dx.doi.org/10.1126/science.1236566; PMID: 23888043
- Gibbons JJ, Abraham RT, Yu K. Mammalian target of rapamycin: discovery of rapamycin reveals a signaling pathway important for normal and cancer cell growth. Semin Oncol 2009; 36:Suppl 3 S3 - 17; http://dx.doi.org/10.1053/j.seminoncol.2009.10.011; PMID: 19963098
- Zeng Z, Sarbassov D, Samudio IJ, Yee KW, Munsell MF, Ellen Jackson C, Giles FJ, Sabatini DM, Andreeff M, Konopleva M. Rapamycin derivatives reduce mTORC2 signaling and inhibit AKT activation in AML. Blood 2007; 109:3509 - 12; http://dx.doi.org/10.1182/blood-2006-06-030833; PMID: 17179228
- Lamming DW, Ye L, Katajisto P, Goncalves MD, Saitoh M, Stevens DM, Davis JG, Salmon AB, Richardson A, Ahima RS, et al. Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 2012; 335:1638 - 43; http://dx.doi.org/10.1126/science.1215135; PMID: 22461615
- Garber K. Rapamycin’s resurrection: a new way to target the cancer cell cycle. J Natl Cancer Inst 2001; 93:1517 - 9; http://dx.doi.org/10.1093/jnci/93.20.1517; PMID: 11604470
- Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell 2007; 12:9 - 22; http://dx.doi.org/10.1016/j.ccr.2007.05.008; PMID: 17613433
- Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, Staroslawska E, Sosman J, McDermott D, Bodrogi I, et al, Global ARCC Trial. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med 2007; 356:2271 - 81; http://dx.doi.org/10.1056/NEJMoa066838; PMID: 17538086
- Faivre S, Kroemer G, Raymond E. Current development of mTOR inhibitors as anticancer agents. Nat Rev Drug Discov 2006; 5:671 - 88; http://dx.doi.org/10.1038/nrd2062; PMID: 16883305
- Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer 2004; 4:335 - 48; http://dx.doi.org/10.1038/nrc1362; PMID: 15122205
- Chiarini F, Lonetti A, Teti G, Orsini E, Bressanin D, Cappellini A, Ricci F, Tazzari PL, Ognibene A, Falconi M, et al. A combination of temsirolimus, an allosteric mTOR inhibitor, with clofarabine as a new therapeutic option for patients with acute myeloid leukemia. Oncotarget 2012; 3:1615 - 28; PMID: 23271044
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011; 12:21 - 35; http://dx.doi.org/10.1038/nrm3025; PMID: 21157483
- Janes MR, Fruman DA. Targeting TOR dependence in cancer. Oncotarget 2010; 1:69 - 76; PMID: 20657741
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev 2013; 23:53 - 62; http://dx.doi.org/10.1016/j.gde.2012.12.005; PMID: 23317514
- Altman JK, Sassano A, Platanias LC. Targeting mTOR for the treatment of AML. New agents and new directions. Oncotarget 2011; 2:510 - 7; PMID: 21680954
- Janku F, Wheler JJ, Naing A, Stepanek VM, Falchook GS, Fu S, Garrido-Laguna I, Tsimberidou AM, Piha-Paul SA, Moulder SL, et al. PIK3CA mutations in advanced cancers: characteristics and outcomes. Oncotarget 2012; 3:1566 - 75; PMID: 23248156
- Law BK. Rapamycin: an anti-cancer immunosuppressant?. Crit Rev Oncol Hematol 2005; 56:47 - 60; http://dx.doi.org/10.1016/j.critrevonc.2004.09.009; PMID: 16039868
- Markman B, Dienstmann R, Tabernero J. Targeting the PI3K/Akt/mTOR pathway--beyond rapalogs. Oncotarget 2010; 1:530 - 43; PMID: 21317449
- Blagosklonny MV. Prevention of cancer by inhibiting aging. Cancer Biol Ther 2008; 7:1520 - 4; http://dx.doi.org/10.4161/cbt.7.10.6663; PMID: 18769112
- Blagosklonny MV. Rapalogs in cancer prevention: anti-aging or anticancer?. Cancer Biol Ther 2012; 13:1349 - 54; http://dx.doi.org/10.4161/cbt.22859; PMID: 23151465
- Sharp ZD. Aging and TOR: interwoven in the fabric of life. Cell Mol Life Sci 2011; 68:587 - 97; http://dx.doi.org/10.1007/s00018-010-0542-0; PMID: 20960025
- DePinho RA. The age of cancer. Nature 2000; 408:248 - 54; http://dx.doi.org/10.1038/35041694; PMID: 11089982
- Anisimov VN, Berstein LM, Popovich IG, Zabezhinski MA, Egormin PA, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Kovalenko IG, et al. If started early in life, metformin treatment increases life span and postpones tumors in female SHR mice. Aging (Albany NY) 2011; 3:148 - 57; PMID: 21386129
- Berstein LM. Metformin in obesity, cancer and aging: addressing controversies. Aging (Albany NY) 2012; 4:320 - 9; PMID: 22589237
- Ikeno Y, Bronson RT, Hubbard GB, Lee SB, Bartke A. Delayed occurrence of fatal neoplastic diseases in ames dwarf mice: correlation to extended longevity. J Gerontol A Biol Sci Med Sci 2003; 58:291 - 6; http://dx.doi.org/10.1093/gerona/58.4.B291; PMID: 12663691
- Berrigan D, Perkins SN, Haines DC, Hursting SD. Adult-onset calorie restriction and fasting delay spontaneous tumorigenesis in p53-deficient mice. Carcinogenesis 2002; 23:817 - 22; http://dx.doi.org/10.1093/carcin/23.5.817; PMID: 12016155
- Longo VD, Fontana L. Calorie restriction and cancer prevention: metabolic and molecular mechanisms. Trends Pharmacol Sci 2010; 31:89 - 98; http://dx.doi.org/10.1016/j.tips.2009.11.004; PMID: 20097433
- Blagosklonny MV. Cell senescence and hypermitogenic arrest. EMBO Rep 2003; 4:358 - 62; http://dx.doi.org/10.1038/sj.embor.embor806; PMID: 12671679
- Blagosklonny MV. Cell cycle arrest is not yet senescence, which is not just cell cycle arrest: terminology for TOR-driven aging. Aging (Albany NY) 2012; 4:159 - 65; PMID: 22394614
- Blagosklonny MV, Hall MN. Growth and aging: a common molecular mechanism. Aging (Albany NY) 2009; 1:357 - 62; PMID: 20157523
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell 2006; 124:471 - 84; http://dx.doi.org/10.1016/j.cell.2006.01.016; PMID: 16469695
- Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell 2010; 40:310 - 22; http://dx.doi.org/10.1016/j.molcel.2010.09.026; PMID: 20965424
- Loewith R, Hall MN. Target of rapamycin (TOR) in nutrient signaling and growth control. Genetics 2011; 189:1177 - 201; http://dx.doi.org/10.1534/genetics.111.133363; PMID: 22174183
- Hands SL, Proud CG, Wyttenbach A. mTOR’s role in ageing: protein synthesis or autophagy?. Aging (Albany NY) 2009; 1:586 - 97; PMID: 20157541
- Demaria M, Campisi J. Matters of life and breath: A role for hypoxia in determining cell state. Aging (Albany NY) 2012; 4:523 - 4; PMID: 22915708
- Blagosklonny MV. Hypoxia, MTOR and autophagy: converging on senescence or quiescence. Autophagy 2013; 9:260 - 2; http://dx.doi.org/10.4161/auto.22783; PMID: 23192222
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 2008; 6:2853 - 68; http://dx.doi.org/10.1371/journal.pbio.0060301; PMID: 19053174
- Demidenko ZN, Blagosklonny MV. Quantifying pharmacologic suppression of cellular senescence: prevention of cellular hypertrophy versus preservation of proliferative potential. Aging (Albany NY) 2009; 1:1008 - 16; PMID: 20157583
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell 2013; 153:1194 - 217; http://dx.doi.org/10.1016/j.cell.2013.05.039; PMID: 23746838
- Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 2013; 123:966 - 72; http://dx.doi.org/10.1172/JCI64098; PMID: 23454759
- Leontieva OV, Lenzo F, Demidenko ZN, Blagosklonny MV. Hyper-mitogenic drive coexists with mitotic incompetence in senescent cells. Cell Cycle 2012; 11:4642 - 9; http://dx.doi.org/10.4161/cc.22937; PMID: 23187803
- Leontieva OV, Demidenko ZN, Blagosklonny MV. MEK drives cyclin D1 hyperelevation during geroconversion. Cell Death Differ 2013; 20:1241 - 9; http://dx.doi.org/10.1038/cdd.2013.86; PMID: 23852369
- Demidenko ZN, Zubova SG, Bukreeva EI, Pospelov VA, Pospelova TV, Blagosklonny MV. Rapamycin decelerates cellular senescence. Cell Cycle 2009; 8:1888 - 95; http://dx.doi.org/10.4161/cc.8.12.8606; PMID: 19471117
- Leontieva OV, Blagosklonny MV. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010; 2:924 - 35; PMID: 21212465
- Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A 2010; 107:9660 - 4; http://dx.doi.org/10.1073/pnas.1002298107; PMID: 20457898
- Halicka HD, Zhao H, Li J, Lee YS, Hsieh TC, Wu JM, Darzynkiewicz Z. Potential anti-aging agents suppress the level of constitutive mTOR- and DNA damage- signaling. Aging (Albany NY) 2012; 4:952 - 65; PMID: 23363784
- Leontieva OV, Natarajan V, Demidenko ZN, Burdelya LG, Gudkov AV, Blagosklonny MV. Hypoxia suppresses conversion from proliferative arrest to cellular senescence. Proc Natl Acad Sci U S A 2012; 109:13314 - 8; http://dx.doi.org/10.1073/pnas.1205690109; PMID: 22847439
- Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 2011; 3:1130 - 41; PMID: 22246147
- Chao SK, Horwitz SB, McDaid HM. Insights into 4E-BP1 and p53 mediated regulation of accelerated cell senescence. Oncotarget 2011; 2:89 - 98; PMID: 21399233
- Dulic V. Be quiet and you’ll keep young: does mTOR underlie p53 action in protecting against senescence by favoring quiescence?. Aging (Albany NY) 2011; 3:3 - 4; PMID: 21248373
- Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle 2012; 11:2391 - 401; http://dx.doi.org/10.4161/cc.20683; PMID: 22627671
- Galluzzi L, Kepp O, Kroemer G. TP53 and MTOR crosstalk to regulate cellular senescence. Aging (Albany NY) 2010; 2:535 - 7; PMID: 20876940
- Borrás C, Monleón D, López-Grueso R, Gambini J, Orlando L, Pallardó FV, Santos E, Viña J, Font de Mora J. RasGrf1 deficiency delays aging in mice. Aging (Albany NY) 2011; 3:262 - 76; PMID: 21422498
- Levine AJ, Harris CR, Puzio-Kuter AM. The interfaces between signal transduction pathways: IGF-1/mTor, p53 and the Parkinson Disease pathway. Oncotarget 2012; 3:1301 - 7; PMID: 23211569
- Vigneron A, Vousden KH. p53, ROS and senescence in the control of aging. Aging (Albany NY) 2010; 2:471 - 4; PMID: 20729567
- Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 1997; 88:593 - 602; http://dx.doi.org/10.1016/S0092-8674(00)81902-9; PMID: 9054499
- Serrano M. Dissecting the role of mTOR complexes in cellular senescence. Cell Cycle 2012; 11:2231 - 2; http://dx.doi.org/10.4161/cc.21065; PMID: 22714590
- Poyurovsky MV, Prives C. P53 and aging: A fresh look at an old paradigm. Aging (Albany NY) 2010; 2:380 - 2; PMID: 20657036
- Blagosklonny MV. Tumor suppression by p53 without apoptosis and senescence: conundrum or rapalog-like gerosuppression?. Aging (Albany NY) 2012; 4:450 - 5; PMID: 22869016
- Loayza-Puch F, Drost J, Rooijers K, Lopes R, Elkon R, Agami R. p53 induces transcriptional and translational programs to suppress cell proliferation and growth. Genome Biol 2013; 14:R32; http://dx.doi.org/10.1186/gb-2013-14-4-r32; PMID: 23594524
- Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Viña J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 2007; 448:375 - 9; http://dx.doi.org/10.1038/nature05949; PMID: 17637672
- Feng Z, Hu W, Rajagopal G, Levine AJ. The tumor suppressor p53: cancer and aging. Cell Cycle 2008; 7:842 - 7; http://dx.doi.org/10.4161/cc.7.7.5657; PMID: 18414039
- McGee MD, Day N, Graham J, Melov S. cep-1/p53-dependent dysplastic pathology of the aging C. elegans gonad. Aging (Albany NY) 2012; 4:256 - 69; PMID: 22562940
- Tucci P. Caloric restriction: is mammalian life extension linked to p53?. Aging (Albany NY) 2012; 4:525 - 34; PMID: 22983298
- Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci Signal 2009; 2:ra75; http://dx.doi.org/10.1126/scisignal.2000559; PMID: 19934433
- Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A 2008; 105:19384 - 9; http://dx.doi.org/10.1073/pnas.0810584105; PMID: 19052232
- Stanfel MN, Shamieh LS, Kaeberlein M, Kennedy BK. The TOR pathway comes of age. Biochim Biophys Acta 2009; 1790:1067 - 74; http://dx.doi.org/10.1016/j.bbagen.2009.06.007; PMID: 19539012
- Polymenis M, Kennedy BK. Chronological and replicative lifespan in yeast: do they meet in the middle?. Cell Cycle 2012; 11:3531 - 2; http://dx.doi.org/10.4161/cc.22041; PMID: 22951539
- Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab 2010; 11:453 - 65; http://dx.doi.org/10.1016/j.cmet.2010.05.001; PMID: 20519118
- Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 2004; 14:885 - 90; http://dx.doi.org/10.1016/j.cub.2004.03.059; PMID: 15186745
- Mirisola MG, Longo VD. Conserved role of Ras-GEFs in promoting aging: from yeast to mice. Aging (Albany NY) 2011; 3:340 - 3; PMID: 21732566
- Partridge L, Alic N, Bjedov I, Piper MD. Ageing in Drosophila: the role of the insulin/Igf and TOR signalling network. Exp Gerontol 2011; 46:376 - 81; http://dx.doi.org/10.1016/j.exger.2010.09.003; PMID: 20849947
- Johnson SC, Rabinovitch PS, Kaeberlein M. mTOR is a key modulator of ageing and age-related disease. Nature 2013; 493:338 - 45; http://dx.doi.org/10.1038/nature11861; PMID: 23325216
- Blagosklonny MV. Rapamycin extends life- and health span because it slows aging. Aging (Albany NY) 2013; 5:592 - 8; PMID: 23934728
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 2009; 460:392 - 5; PMID: 19587680
- Miller RA, Harrison DE, Astle CM, Baur JA, Boyd AR, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci 2011; 66:191 - 201; http://dx.doi.org/10.1093/gerona/glq178; PMID: 20974732
- Wilkinson JE, Burmeister L, Brooks SV, Chan CC, Friedline S, Harrison DE, Hejtmancik JF, Nadon N, Strong R, Wood LK, et al. Rapamycin slows aging in mice. Aging Cell 2012; 11:675 - 82; http://dx.doi.org/10.1111/j.1474-9726.2012.00832.x; PMID: 22587563
- Longo VD, Fontana L. Intermittent supplementation with rapamycin as a dietary restriction mimetic. Aging (Albany NY) 2011; 3:1039 - 40; PMID: 22147496
- Ye L, Widlund AL, Sims CA, Lamming DW, Guan Y, Davis JG, Sabatini DM, Harrison DE, Vang O, Baur JA. Rapamycin doses sufficient to extend lifespan do not compromise muscle mitochondrial content or endurance. Aging (Albany NY) 2013; 5:539 - 50; PMID: 23929887
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle 2011; 10:4230 - 6; http://dx.doi.org/10.4161/cc.10.24.18486; PMID: 22107964
- Del Barco S, Vazquez-Martin A, Cufí S, Oliveras-Ferraros C, Bosch-Barrera J, Joven J, Martin-Castillo B, Menendez JA. Metformin: multi-faceted protection against cancer. Oncotarget 2011; 2:896 - 917; PMID: 22203527
- Anisimov VN, Piskunova TS, Popovich IG, Zabezhinski MA, Tyndyk ML, Egormin PA, Yurova MV, Rosenfeld SV, Semenchenko AV, Kovalenko IG, et al. Gender differences in metformin effect on aging, life span and spontaneous tumorigenesis in 129/Sv mice. Aging (Albany NY) 2010; 2:945 - 58; PMID: 21164223
- Anisimov VN. Metformin for aging and cancer prevention. Aging (Albany NY) 2010; 2:760 - 74; PMID: 21084729
- Anisimov VN, Egormin PA, Piskunova TS, Popovich IG, Tyndyk ML, Yurova MN, Zabezhinski MA, Anikin IV, Karkach AS, Romanyukha AA. Metformin extends life span of HER-2/neu transgenic mice and in combination with melatonin inhibits growth of transplantable tumors in vivo. Cell Cycle 2010; 9:188 - 97; http://dx.doi.org/10.4161/cc.9.1.10407; PMID: 20016287
- Blagosklonny MV. Answering the ultimate question “what is the proximal cause of aging?”. Aging (Albany NY) 2012; 4:861 - 77; PMID: 23425777
- Kolosova NG, Muraleva NA, Zhdankina AA, Stefanova NA, Fursova AZ, Blagosklonny MV. Prevention of age-related macular degeneration-like retinopathy by rapamycin in rats. Am J Pathol 2012; 181:472 - 7; http://dx.doi.org/10.1016/j.ajpath.2012.04.018; PMID: 22683466
- Zhao C, Vollrath D. mTOR pathway activation in age-related retinal disease. Aging (Albany NY) 2011; 3:346 - 7; PMID: 21483039
- Tsang CK, Qi H, Liu LF, Zheng XFS. Targeting mammalian target of rapamycin (mTOR) for health and diseases. Drug Discov Today 2007; 12:112 - 24; http://dx.doi.org/10.1016/j.drudis.2006.12.008; PMID: 17275731
- Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 2013; 12:851 - 62; http://dx.doi.org/10.1111/acel.12109; PMID: 23734717
- Blagosklonny MV. Hormesis does not make sense except in the light of TOR-driven aging. Aging (Albany NY) 2011; 3:1051 - 62; PMID: 22166724
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr Opin Genet Dev 2013; 23:53 - 62; http://dx.doi.org/10.1016/j.gde.2012.12.005; PMID: 23317514
- Halloran J, Hussong SA, Burbank R, Podlutskaya N, Fischer KE, Sloane LB, Austad SN, Strong R, Richardson A, Hart MJ, et al. Chronic inhibition of mammalian target of rapamycin by rapamycin modulates cognitive and non-cognitive components of behavior throughout lifespan in mice. Neuroscience 2012; 223:102 - 13; http://dx.doi.org/10.1016/j.neuroscience.2012.06.054; PMID: 22750207
- Dazert E, Hall MN. mTOR signaling in disease. Curr Opin Cell Biol 2011; 23:744 - 55; http://dx.doi.org/10.1016/j.ceb.2011.09.003; PMID: 21963299
- Blagosklonny MV. Prospective treatment of age-related diseases by slowing down aging. Am J Pathol 2012; 181:1142 - 6; http://dx.doi.org/10.1016/j.ajpath.2012.06.024; PMID: 22841821
- Blagosklonny MV. Once again on rapamycin-induced insulin resistance and longevity: despite of or owing to. Aging (Albany NY) 2012; 4:350 - 8; PMID: 22683661
- Blagosklonny MV. How to save Medicare: the anti-aging remedy. Aging (Albany NY) 2012; 4:547 - 52; PMID: 22915707
- Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant 2004; 18:446 - 9; http://dx.doi.org/10.1111/j.1399-0012.2004.00188.x; PMID: 15233824
- Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation 2005; 80:883 - 9; http://dx.doi.org/10.1097/01.TP.0000184006.43152.8D; PMID: 16249734
- Yakupoglu YK, Buell JF, Woodle S, Kahan BD. Individualization of immunosuppressive therapy. III. Sirolimus associated with a reduced incidence of malignancy. Transplant Proc 2006; 38:358 - 61; http://dx.doi.org/10.1016/j.transproceed.2006.01.019; PMID: 16549120
- Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L, et al. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol 2006; 17:581 - 9; http://dx.doi.org/10.1681/ASN.2005090993; PMID: 16434506
- Alberú J, Pascoe MD, Campistol JM, Schena FP, Rial MdelC, Polinsky M, Neylan JF, Korth-Bradley J, Goldberg-Alberts R, Maller ES, Sirolimus CONVERT Trial Study Group. Lower malignancy rates in renal allograft recipients converted to sirolimus-based, calcineurin inhibitor-free immunotherapy: 24-month results from the CONVERT trial. Transplantation 2011; 92:303 - 10; http://dx.doi.org/10.1097/TP.0b013e3182247ae2; PMID: 21792049
- Mohsin N, Budruddin M, Pakkyara A, Darweesh A, Nayyer M, Amitabh J, Daar AS. Complete regression of visceral Kaposi’s sarcoma after conversion to sirolimus. Exp Clin Transplant 2005; 3:366 - 9; PMID: 16417445
- Zmonarski SC, Boratyńska M, Rabczyński J, Kazimierczak K, Klinger M. Regression of Kaposi’s sarcoma in renal graft recipients after conversion to sirolimus treatment. Transplant Proc 2005; 37:964 - 6; http://dx.doi.org/10.1016/j.transproceed.2004.12.172; PMID: 15848592
- Rizell M, Cahlin C, Friman S, Hafström L, Lönn L, Olausson M, Lindner P. Impressive regression of primary liver cancer after treatment with sirolimus. Acta Oncol 2005; 44:496; http://dx.doi.org/10.1080/02841860510044610; PMID: 16118084
- Cullis B, D’Souza R, McCullagh P, Harries S, Nicholls A, Lee R, Bingham C. Sirolimus-induced remission of posttransplantation lymphoproliferative disorder. Am J Kidney Dis 2006; 47:e67 - 72; http://dx.doi.org/10.1053/j.ajkd.2006.01.029; PMID: 16632009
- Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi’s sarcoma in renal-transplant recipients. N Engl J Med 2005; 352:1317 - 23; http://dx.doi.org/10.1056/NEJMoa042831; PMID: 15800227
- Mártinez JM, Pulido LB, Bellido CB, Usero DD, Aguilar LT, Moreno JL, Artacho GS, Díez-Canedo JS, Gómez LM, Bravo MA. Rescue immunosuppression with mammalian target of rapamycin inhibitor drugs in liver transplantation. Transplant Proc 2010; 42:641 - 3; http://dx.doi.org/10.1016/j.transproceed.2010.02.011; PMID: 20304212
- Euvrard S, Morelon E, Rostaing L, Goffin E, Brocard A, Tromme I, Broeders N, del Marmol V, Chatelet V, Dompmartin A, et al, TUMORAPA Study Group. Sirolimus and secondary skin-cancer prevention in kidney transplantation. N Engl J Med 2012; 367:329 - 39; http://dx.doi.org/10.1056/NEJMoa1204166; PMID: 22830463
- Salgo R, Gossmann J, Schöfer H, Kachel HG, Kuck J, Geiger H, Kaufmann R, Scheuermann EH. Switch to a sirolimus-based immunosuppression in long-term renal transplant recipients: reduced rate of (pre-)malignancies and nonmelanoma skin cancer in a prospective, randomized, assessor-blinded, controlled clinical trial. Am J Transplant 2010; 10:1385 - 93; http://dx.doi.org/10.1111/j.1600-6143.2009.02997.x; PMID: 20121752
- Campbell SB, Walker R, Tai SS, Jiang Q, Russ GR. Randomized controlled trial of sirolimus for renal transplant recipients at high risk for nonmelanoma skin cancer. Am J Transplant 2012; 12:1146 - 56; http://dx.doi.org/10.1111/j.1600-6143.2012.04004.x; PMID: 22420843
- Liu M, Howes A, Lesperance J, Stallcup WB, Hauser CA, Kadoya K, Oshima RG, Abraham RT. Antitumor activity of rapamycin in a transgenic mouse model of ErbB2-dependent human breast cancer. Cancer Res 2005; 65:5325 - 36; http://dx.doi.org/10.1158/0008-5472.CAN-04-4589; PMID: 15958580
- Mabuchi S, Altomare DA, Connolly DC, Klein-Szanto A, Litwin S, Hoelzle MK, Hensley HH, Hamilton TC, Testa JR. RAD001 (Everolimus) delays tumor onset and progression in a transgenic mouse model of ovarian cancer. Cancer Res 2007; 67:2408 - 13; http://dx.doi.org/10.1158/0008-5472.CAN-06-4490; PMID: 17363557
- Granville CA, Warfel N, Tsurutani J, Hollander MC, Robertson M, Fox SD, Veenstra TD, Issaq HJ, Linnoila RI, Dennis PA. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin Cancer Res 2007; 13:2281 - 9; http://dx.doi.org/10.1158/1078-0432.CCR-06-2570; PMID: 17404113
- Robinson J, Lai C, Martin A, Nye E, Tomlinson I, Silver A. Oral rapamycin reduces tumour burden and vascularization in Lkb1(+/-) mice. J Pathol 2009; 219:35 - 40; http://dx.doi.org/10.1002/path.2562; PMID: 19434632
- Komarova EA, Antoch MP, Novototskaya LR, Chernova OB, Paszkiewicz G, Leontieva OV, Blagosklonny MV, Gudkov AV. Rapamycin extends lifespan and delays tumorigenesis in heterozygous p53+/- mice. Aging (Albany NY) 2012; 4:709 - 14; PMID: 23123616
- Comas M, Toshkov I, Kuropatwinski KK, Chernova OB, Polinsky A, Blagosklonny MV, Gudkov AV, Antoch MP. New nanoformulation of rapamycin Rapatar extends lifespan in homozygous p53-/- mice by delaying carcinogenesis. Aging (Albany NY) 2012; 4:715 - 22; PMID: 23117593
- Donehower LA. Rapamycin as longevity enhancer and cancer preventative agent in the context of p53 deficiency. Aging (Albany NY) 2012; 4:660 - 1; PMID: 23128359
- Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Antoch MP, Blagosklonny MV. Rapamycin extends maximal lifespan in cancer-prone mice. Am J Pathol 2010; 176:2092 - 7; http://dx.doi.org/10.2353/ajpath.2010.091050; PMID: 20363920
- Thiem S, Pierce TP, Palmieri M, Putoczki TL, Buchert M, Preaudet A, Farid RO, Love C, Catimel B, Lei Z, et al. mTORC1 inhibition restricts inflammation-associated gastrointestinal tumorigenesis in mice. J Clin Invest 2013; 123:767 - 81; PMID: 23321674
- Major P. Potential of mTOR inhibitors for the treatment of subependymal giant cell astrocytomas in tuberous sclerosis complex. Aging (Albany NY) 2011; 3:189 - 91; PMID: 21415462
- Checkley LA, Rho O, Moore T, Hursting S, DiGiovanni J. Rapamycin is a potent inhibitor of skin tumor promotion by 12-O-tetradecanoylphorbol-13-acetate. Cancer Prev Res (Phila) 2011; 4:1011 - 20; http://dx.doi.org/10.1158/1940-6207.CAPR-10-0375; PMID: 21733825
- Athar M, Kopelovich L. Rapamycin and mTORC1 inhibition in the mouse: skin cancer prevention. Cancer Prev Res (Phila) 2011; 4:957 - 61; http://dx.doi.org/10.1158/1940-6207.CAPR-11-0266; PMID: 21733819
- Khanna A, Kapahi P. Rapamycin: killing two birds with one stone. Aging (Albany NY) 2011; 3:1043 - 4; PMID: 22170738
- Livi CB, Hardman RL, Christy BA, Dodds SG, Jones D, Williams C, Strong R, Bokov A, Javors MA, Ikeno Y, et al. Rapamycin extends life span of Rb1+/- mice by inhibiting neuroendocrine tumors. Aging (Albany NY) 2013; 5:100 - 10; PMID: 23454836
- Liu Y, Huang Y, Wang Z, Huang Y, Li X, Louie A, Wei G, Mao JH. Temporal mTOR inhibition protects Fbxw7-deficient mice from radiation-induced tumor development. Aging (Albany NY) 2013; 5:111 - 9; PMID: 23454868
- Danilov A, Shaposhnikov M, Plyusnina E, Kogan V, Fedichev P, Moskalev A. Selective anticancer agents suppress aging in Drosophila. Oncotarget 2013; 4:1507 - 26; PMID: 24096697
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell 2000; 100:57 - 70; http://dx.doi.org/10.1016/S0092-8674(00)81683-9; PMID: 10647931
- Weigelt B, Warne PH, Downward J. PIK3CA mutation, but not PTEN loss of function, determines the sensitivity of breast cancer cells to mTOR inhibitory drugs. Oncogene 2011; 30:3222 - 33; http://dx.doi.org/10.1038/onc.2011.42; PMID: 21358673
- Alain T, Sonenberg N, Topisirovic I. mTOR inhibitor efficacy is determined by the eIF4E/4E-BP ratio. Oncotarget 2012; 3:1491 - 2; PMID: 23455427
- Choo AY, Blenis J. TORgeting oncogene addiction for cancer therapy. Cancer Cell 2006; 9:77 - 9; http://dx.doi.org/10.1016/j.ccr.2006.01.021; PMID: 16473275
- Floc’h N, Abate-Shen C. The promise of dual targeting Akt/mTOR signaling in lethal prostate cancer. Oncotarget 2012; 3:1483 - 4; PMID: 23242005
- McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Franklin RA, Montalto G, Cervello M, Libra M, Candido S, Malaponte G, et al. Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance. Oncotarget 2012; 3:1068 - 111; PMID: 23085539
- Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol 2009; 27:2278 - 87; http://dx.doi.org/10.1200/JCO.2008.20.0766; PMID: 19332717
- Bressanin D, Evangelisti C, Ricci F, Tabellini G, Chiarini F, Tazzari PL, Melchionda F, Buontempo F, Pagliaro P, Pession A, et al. Harnessing the PI3K/Akt/mTOR pathway in T-cell acute lymphoblastic leukemia: eliminating activity by targeting at different levels. Oncotarget 2012; 3:811 - 23; PMID: 22885370
- Martelli AM, Chiarini F, Evangelisti C, Cappellini A, Buontempo F, Bressanin D, Fini M, McCubrey JA. Two hits are better than one: targeting both phosphatidylinositol 3-kinase and mammalian target of rapamycin as a therapeutic strategy for acute leukemia treatment. Oncotarget 2012; 3:371 - 94; PMID: 22564882
- Garrett JT, Chakrabarty A, Arteaga CL. Will PI3K pathway inhibitors be effective as single agents in patients with cancer?. Oncotarget 2011; 2:1314 - 21; PMID: 22248929
- Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol 2009; 27:2630 - 7; http://dx.doi.org/10.1200/JCO.2008.18.8391; PMID: 19380449
- Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 2012; 366:520 - 9; http://dx.doi.org/10.1056/NEJMoa1109653; PMID: 22149876
- Blagosklonny MV, Darzynkiewicz Z. Four birds with one stone: RAPA as potential anticancer therapy. Cancer Biol Ther 2002; 1:359 - 61; http://dx.doi.org/10.4161/cbt.1.4.6; PMID: 12432246
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci U S A 2001; 98:12072 - 7; http://dx.doi.org/10.1073/pnas.211053698; PMID: 11593017
- Martens JW, Sieuwerts AM, Bolt-deVries J, Bosma PT, Swiggers SJ, Klijn JG, Foekens JA. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemost 2003; 89:393 - 404; PMID: 12574821
- Lewis DA, Travers JB, Machado C, Somani AK, Spandau DF. Reversing the aging stromal phenotype prevents carcinoma initiation. Aging (Albany NY) 2011; 3:407 - 16; PMID: 21515933
- Mercier I, Camacho J, Titchen K, Gonzales DM, Quann K, Bryant KG, Molchansky A, Milliman JN, Whitaker-Menezes D, Sotgia F, et al. Caveolin-1 and accelerated host aging in the breast tumor microenvironment: chemoprevention with rapamycin, an mTOR inhibitor and anti-aging drug. Am J Pathol 2012; 181:278 - 93; http://dx.doi.org/10.1016/j.ajpath.2012.03.017; PMID: 22698676
- Apontes P, Leontieva OV, Demidenko ZN, Li F, Blagosklonny MV. Exploring long-term protection of normal human fibroblasts and epithelial cells from chemotherapy in cell culture. Oncotarget 2011; 2:222 - 33; PMID: 21447859
- van Leeuwen IM, Laín S. Pharmacological manipulation of the cell cycle and metabolism to protect normal tissues against conventional anticancer drugs. Oncotarget 2011; 2:274 - 6; PMID: 21512204
- Iglesias-Bartolome R, Patel V, Cotrim A, Leelahavanichkul K, Molinolo AA, Mitchell JB, Gutkind JS. mTOR inhibition prevents epithelial stem cell senescence and protects from radiation-induced mucositis. Cell Stem Cell 2012; 11:401 - 14; http://dx.doi.org/10.1016/j.stem.2012.06.007; PMID: 22958932
- Iglesias-Bartolome R, Gutkind SJ. Exploiting the mTOR paradox for disease prevention. Oncotarget 2012; 3:1061 - 3; PMID: 23165441
- Blagosklonny MV. Common drugs and treatments for cancer and age-related diseases: revitalizing answers to NCI’s provocative questions. Oncotarget 2012; 3:1711 - 24; PMID: 23565531
- Leontieva OV, Paszkiewicz G, Demidenko ZN, Blagosklonny MV. Resveratrol potentiates rapamycin to prevent hyperinsulinemia and obesity in male mice on high fat diet. Cell Death Dis 2013; 4:e472; http://dx.doi.org/10.1038/cddis.2012.202; PMID: 23348586
- Caruso C, Lio D, Cavallone L, Franceschi C. Aging, longevity, inflammation, and cancer. Ann N Y Acad Sci 2004; 1028:1 - 13; http://dx.doi.org/10.1196/annals.1322.001; PMID: 15915584
- Gudkov AV, Gurova KV, Komarova EA. Inflammation and p53: A Tale of Two Stresses. Genes Cancer 2011; 2:503 - 16; http://dx.doi.org/10.1177/1947601911409747; PMID: 21779518
- Salminen A, Kaarniranta K, Kauppinen A. Inflammaging: disturbed interplay between autophagy and inflammasomes. Aging (Albany NY) 2012; 4:166 - 75; PMID: 22411934
- Howcroft TK, Campisi J, Louis GB, Smith MT, Wise B, Wyss-Coray T, Augustine AD, McElhaney JE, Kohanski R, Sierra F. The role of inflammation in age-related disease. Aging (Albany NY) 2013; 5:84 - 93; PMID: 23474627
- Turner AP, Shaffer VO, Araki K, Martens C, Turner PL, Gangappa S, Ford ML, Ahmed R, Kirk AD, Larsen CP. Sirolimus enhances the magnitude and quality of viral-specific CD8+ T-cell responses to vaccinia virus vaccination in rhesus macaques. Am J Transplant 2011; 11:613 - 8; http://dx.doi.org/10.1111/j.1600-6143.2010.03407.x; PMID: 21342450
- Hill JA, Hummel M, Starling RC, Kobashigawa JA, Perrone SV, Arizón JM, Simonsen S, Abeywickrama KH, Bara C. A lower incidence of cytomegalovirus infection in de novo heart transplant recipients randomized to everolimus. Transplantation 2007; 84:1436 - 42; http://dx.doi.org/10.1097/01.tp.0000290686.68910.bd; PMID: 18091519
- Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature 2009; 460:108 - 12; http://dx.doi.org/10.1038/nature08155; PMID: 19543266
- Wang Y, Wang XY, Subjeck JR, Shrikant PA, Kim HL. Temsirolimus, an mTOR inhibitor, enhances anti-tumour effects of heat shock protein cancer vaccines. Br J Cancer 2011; 104:643 - 52; http://dx.doi.org/10.1038/bjc.2011.15; PMID: 21285988
- Ferrer IR, Wagener ME, Robertson JM, Turner AP, Araki K, Ahmed R, Kirk AD, Larsen CP, Ford ML. Cutting edge: Rapamycin augments pathogen-specific but not graft-reactive CD8+ T cell responses. J Immunol 2010; 185:2004 - 8; http://dx.doi.org/10.4049/jimmunol.1001176; PMID: 20631309
- Hinojosa CA, Mgbemena V, Van Roekel S, Austad SN, Miller RA, Bose S, Orihuela CJ. Enteric-delivered rapamycin enhances resistance of aged mice to pneumococcal pneumonia through reduced cellular senescence. Exp Gerontol 2012; 47:958 - 65; http://dx.doi.org/10.1016/j.exger.2012.08.013; PMID: 22981852
- Senovilla L, Vitale I, Martins I, Kepp O, Galluzzi L, Zitvogel L, Castedo M, Kroemer G. An anticancer therapy-elicited immunosurveillance system that eliminates tetraploid cells. Oncoimmunology 2013; 2:e22409; http://dx.doi.org/10.4161/onci.22409; PMID: 23482968
- Boilève A, Senovilla L, Vitale I, Lissa D, Martins I, Métivier D, van den Brink S, Clevers H, Galluzzi L, Castedo M, et al. Immunosurveillance against tetraploidization-induced colon tumorigenesis. Cell Cycle 2013; 12:473 - 9; http://dx.doi.org/10.4161/cc.23369; PMID: 23324343
- Yevsa T, Kang TW, Zender L. Immune surveillance of pre-cancerous senescent hepatocytes limits hepatocellular carcinoma development. Oncoimmunology 2012; 1:398 - 9; http://dx.doi.org/10.4161/onci.19128; PMID: 22737629
- Prendergast GC, Metz R. A perspective on new immune adjuvant principles: Reprogramming inflammatory states to permit clearance of cancer cells and other age-associated cellular pathologies. Oncoimmunology 2012; 1:924 - 9; http://dx.doi.org/10.4161/onci.21358; PMID: 23162760
- Ho CC, Hau PM, Marxer M, Poon RY. The requirement of p53 for maintaining chromosomal stability during tetraploidization. Oncotarget 2010; 1:583 - 95; PMID: 21317454
- Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity?. Nat Cell Biol 2010; 12:842 - 6; http://dx.doi.org/10.1038/ncb0910-842; PMID: 20811357
- Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 2010; 1:e10; http://dx.doi.org/10.1038/cddis.2009.8; PMID: 21364612
- Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell 2011; 146:682 - 95; http://dx.doi.org/10.1016/j.cell.2011.07.030; PMID: 21884931
- Fujii S, Hara H, Araya J, Takasaka N, Kojima J, Ito S, Minagawa S, Yumino Y, Ishikawa T, Numata T, et al. Insufficient autophagy promotes bronchial epithelial cell senescence in chronic obstructive pulmonary disease. Oncoimmunology 2012; 1:630 - 41; http://dx.doi.org/10.4161/onci.20297; PMID: 22934255
- Vacchelli E, Senovilla L, Eggermont A, Fridman WH, Galon J, Zitvogel L, Kroemer G, Galluzzi L. Trial watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 2013; 2:e23510; http://dx.doi.org/10.4161/onci.23510; PMID: 23687621
- Srivastava RK, Utley A, Shrikant PA. Rapamycin: A rheostat for CD8(+) T-cell-mediated tumor therapy. Oncoimmunology 2012; 1:1189 - 90; http://dx.doi.org/10.4161/onci.20663; PMID: 23170275
- Thomas DL, Doty R, Tosic V, Liu J, Kranz DM, McFadden G, Macneill AL, Roy EJ. Myxoma virus combined with rapamycin treatment enhances adoptive T cell therapy for murine melanoma brain tumors. Cancer Immunol Immunother 2011; 60:1461 - 72; http://dx.doi.org/10.1007/s00262-011-1045-z; PMID: 21656158
- Amiel E, Everts B, Freitas TC, King IL, Curtis JD, Pearce EL, Pearce EJ. Inhibition of mechanistic target of rapamycin promotes dendritic cell activation and enhances therapeutic autologous vaccination in mice. J Immunol 2012; 189:2151 - 8; http://dx.doi.org/10.4049/jimmunol.1103741; PMID: 22826320
- Willcocks LC, Chaudhry AN, Smith JC, Ojha S, Doffinger R, Watson CJ, Smith KG. The effect of sirolimus therapy on vaccine responses in transplant recipients. Am J Transplant 2007; 7:2006 - 11; http://dx.doi.org/10.1111/j.1600-6143.2007.01869.x; PMID: 17578505
- Yi Y, Zhou Z, Shu S, Fang Y, Twitty C, Hilton TL, Aung S, Urba WJ, Fox BA, Hu HM, et al. Autophagy-assisted antigen cross-presentation: Autophagosome as the argo of shared tumor-specific antigens and DAMPs. Oncoimmunology 2012; 1:976 - 8; http://dx.doi.org/10.4161/onci.20059; PMID: 23162777
- Ma Y, Galluzzi L, Zitvogel L, Kroemer G. Autophagy and cellular immune responses. Immunity 2013; 39:211 - 27; http://dx.doi.org/10.1016/j.immuni.2013.07.017; PMID: 23973220
- Ma Y, Adjemian S, Yang H, Catani JP, Hannani D, Martins I, Michaud M, Kepp O, Sukkurwala AQ, Vacchelli E, et al. ATP-dependent recruitment, survival and differentiation of dendritic cell precursors in the tumor bed after anticancer chemotherapy. Oncoimmunology 2013; 2:e24568; http://dx.doi.org/10.4161/onci.24568; PMID: 23894718
- Kepp O, Menger L, Vacchelli E, Adjemian S, Martins I, Ma Y, Sukkurwala AQ, Michaud M, Galluzzi L, Zitvogel L, et al. Anticancer activity of cardiac glycosides: At the frontier between cell-autonomous and immunological effects. Oncoimmunology 2012; 1:1640 - 2; http://dx.doi.org/10.4161/onci.21684; PMID: 23264921
- Metz R, Rust S, Duhadaway JB, Mautino MR, Munn DH, Vahanian NN, Link CJ, Prendergast GC. IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan. Oncoimmunology 2012; 1:1460 - 8; http://dx.doi.org/10.4161/onci.21716; PMID: 23264892
- Sánchez Fructuoso AI, Calvo N, Perez-Flores I, Valero R, Rodríguez-Sánchez B, García de Viedma D, Muñoz P, Barrientos A. Mammalian target of rapamycin signal inhibitors could play a role in the treatment of BK polyomavirus nephritis in renal allograft recipients. Transpl Infect Dis 2011; 13:584 - 91; http://dx.doi.org/10.1111/j.1399-3062.2011.00649.x; PMID: 21585634
- Donia M, McCubrey JA, Bendtzen K, Nicoletti F. Potential use of rapamycin in HIV infection. Br J Clin Pharmacol 2010; 70:784 - 93; http://dx.doi.org/10.1111/j.1365-2125.2010.03735.x; PMID: 21175433
- Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C, et al. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc 2008; 40:1407 - 10; http://dx.doi.org/10.1016/j.transproceed.2008.03.084; PMID: 18589118
- Ozaki KS, Câmara NO, Nogueira E, Pereira MG, Granato C, Melaragno C, Camargo LF, Pacheco-Silva A. The use of sirolimus in ganciclovir-resistant cytomegalovirus infections in renal transplant recipients. Clin Transplant 2007; 21:675 - 80; http://dx.doi.org/10.1111/j.1399-0012.2007.00699.x; PMID: 17845644
- Marty FM, Bryar J, Browne SK, Schwarzberg T, Ho VT, Bassett IV, Koreth J, Alyea EP, Soiffer RJ, Cutler CS, et al. Sirolimus-based graft-versus-host disease prophylaxis protects against cytomegalovirus reactivation after allogeneic hematopoietic stem cell transplantation: a cohort analysis. Blood 2007; 110:490 - 500; http://dx.doi.org/10.1182/blood-2007-01-069294; PMID: 17392502
- McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O’Leary JG, Davis GL, Levy MF, et al. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant 2011; 11:2379 - 87; http://dx.doi.org/10.1111/j.1600-6143.2011.03767.x; PMID: 21967703
- Shahidi S, Moeinzadeh F, Mohammadi M, Gholamrezaei A. Sirolimus-based immunosuppression for treatment of cutaneous warts in kidney transplant recipients. Iran J Kidney Dis 2011; 5:351 - 3; PMID: 21876315
- Krams SM, Martinez OM. Epstein-Barr virus, rapamycin, and host immune responses. Curr Opin Organ Transplant 2008; 13:563 - 8; http://dx.doi.org/10.1097/MOT.0b013e3283186ba9; PMID: 19060543
- Vaysberg M, Balatoni CE, Nepomuceno RR, Krams SM, Martinez OM. Rapamycin inhibits proliferation of Epstein-Barr virus-positive B-cell lymphomas through modulation of cell-cycle protein expression. Transplantation 2007; 83:1114 - 21; http://dx.doi.org/10.1097/01.tp.0000260142.38619.9c; PMID: 17452903
- Toh HC, Teo M, Ong KW, Lee V, Chan E, Lee AS, Vathsala A. Use of sirolimus for Epstein-Barr virus-positive smooth-muscle tumour. Lancet Oncol 2006; 7:955 - 7; http://dx.doi.org/10.1016/S1470-2045(06)70943-3; PMID: 17081922
- Johnsen JI, Baryawno N, Söderberg-Nauclér C. Is human cytomegalovirus a target in cancer therapy?. Oncotarget 2011; 2:1329 - 38; PMID: 22246171
- Söderberg-Nauclér C, Johnsen JI. Cytomegalovirus infection in brain tumors: A potential new target for therapy?. Oncoimmunology 2012; 1:739 - 40; http://dx.doi.org/10.4161/onci.19441; PMID: 22934266
- Strissel PL, Ruebner M, Thiel F, Wachter D, Ekici AB, Wolf F, Thieme F, Ruprecht K, Beckmann MW, Strick R. Reactivation of codogenic endogenous retroviral (ERV) envelope genes in human endometrial carcinoma and prestages: Emergence of new molecular targets. Oncotarget 2012; 3:1204 - 19; PMID: 23085571
- Blagosklonny MV. Oncogenic resistance to growth-limiting conditions. Nat Rev Cancer 2002; 2:221 - 5; http://dx.doi.org/10.1038/nrc743; PMID: 11990858
- Henry CJ, Marusyk A, DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what’s the connection?. Aging (Albany NY) 2011; 3:643 - 56; PMID: 21765201
- Greaves M. Leukemogenesis and ageing: 'fit for transformation'?. Aging (Albany, NY) 2011; 3:79 - 80
- McCullough KD, Coleman WB, Ricketts SL, Wilson JW, Smith GJ, Grisham JW. Plasticity of the neoplastic phenotype in vivo is regulated by epigenetic factors. Proc Natl Acad Sci U S A 1998; 95:15333 - 8; http://dx.doi.org/10.1073/pnas.95.26.15333; PMID: 9860969
- Mikheev AM, Stoll EA, Ramakrishna R, Mikheeva SA, Horner PJ, Rostomily RC. Geropotency: increased malignant potential of aging neural progenitors. Aging (Albany NY) 2012; 4:854 - 5; PMID: 23257545