Abstract
While conventional anticancer therapies, including surgical resection, radiotherapy, and/or chemotherapy, are relatively efficient at eliminating primary tumors, these treatment modalities are largely ineffective against metastases. At least in part, this reflects the rather inefficient delivery of conventional anticancer agents to metastatic lesions. We have recently demonstrated that myeloid-derived suppressor cells (MDSCs) can be used as cellular missiles to selectively deliver a radioisotope-coupled attenuated variant of Listeria monocytogenes to both primary and metastatic neoplastic lesions in mice with pancreatic cancer. This novel immunotherapeutic intervention robustly inhibited tumor growth while promoting a dramatic decrease in the number of metastases.
Myeloid-Derived Suppressor Cells
Myeloid derived suppressor cells (MDSCs) are a heterogeneous population of myeloid progenitor cells, i.e., immature macrophages, granulocytes, and dendritic cells (DCs), that are endowed with a robust immunosuppressive activity.Citation1 In normal healthy individuals, immature myeloid cells differentiate into mature granulocytes, macrophages, and DCs. Conversely, in cancer patients, MDSCs respond to tumor-secreted factors including interleukin (IL)-6, granulocyte macrophage colony-stimulating factor (GM-CSF) and IL-1β by emigrating from the bone marrow and accumulating within primary neoplastic lesions and metastases.Citation2 In the tumor microenvironment (TME), MDSCs are prevented from differentiation and are stimulated to express immunosuppressive enzymes like arginase I and inducible nitric oxide synthetase as well as to produce immunosuppressive mediators, including reactive oxygen species and various cytokines such as IL-6, IL-10, and transforming growth factor β1 (TGFβ1).Citation1,Citation2 Altogether, these enzymes and factors are responsible for the suppression of T-cell and natural killer (NK)-cell responses in the TME.Citation1-Citation3 While MDSCs are highly immunosuppressive,Citation1-Citation3 we have recently shown that these cells can be used as a vehicle to deliver anticancer agents to the TME with help of Listeria monocytogenes.Citation4,Citation5 MDSCs are abundant not only in the peripheral blood of tumor-bearing mice, but also in the circulation of patients affected by almost all types of cancer. Thus, MDSC/Listeria-based interventions might be suitable for the treatment of a wide panel of neoplasms.
Listeria Monocytogenes
Listeria monocytogenes is Gram-positive facultative intracellular bacterium that causes food-poisoning. In contrast to wild type Listeria, attenuated non-virulent strains of Listeria monocytogenes are attractive vaccine vectors because of their unique ability to selectively deliver antigenic determinants to antigen-presenting cells (APCs) such as monocytes, macrophages, and DCs through phagocytosis, while activating strong innate and adaptive immune responses.Citation6Listeria-based anticancer vaccines have been developed and tested by different groups, including ourselves, in animal models of various neoplasms including (but not limited to) breast, pancreatic, cervical, and colorectal carcinoma.Citation4,Citation6,Citation7
We discovered that an attenuated strain of L. monocytogenes (Listeriaat) infects not only APCs but also cancer cells. Malignant cells are efficiently killed by Listeriaat upon the generation of high levels of reactive oxygen species.Citation7 Importantly, Listeriaat appears to multiply within primary neoplastic lesions as well as within metastases, and infected cancer cells become sensitive to the cytotoxic activity of Listeriaat-activated T and NK cells. The selective survival and replication of Listeriaat in malignant, but not in normal, tissues is attributed to the fact that Listeriaat is efficiently cleared by the immune system in non-transformed tissues but not in the heavily immunosuppressed TME.Citation4,Citation5 This raised the question on how Listeriaat could safely reach the TME without being eliminated. It turned out that MDSCs play an important role in the delivery of L. monocytogenes to the TME.Citation4,Citation5
MDSCs Selectively Deliver Listeriaat to Primary Malignant Lesions and Metastases
MDSCs are well known as one of the major contributors to the establishment of an immunosuppressive TME, where they are recruited by chemoattractants produced by malignant cells.Citation2 We discovered a unique relationship between Listeriaat and MDSCs. Listeriaat infects MDSCs and can survive within MDSCs because of their immunosuppressive nature. Moreover, infected MDSCs appear to selectively deliver Listeriaat to primary malignant lesions as well as to metastases. Once in the TME, Listeriaat spreads first from MDSCs to neighboring neoplastic cells, and then from cancer cell to cancer cell through a characteristic mechanism of dissemination.Citation8 These results suggest that MDSCs can be used as cellular missiles to deliver anticancer agents to primary tumors as well as to metastatic lesions.Citation4 We have recently demonstrated that radioisotope-labeled Listeriaat bacteria are selectively delivered by MDSCs to primary tumors and metastases in a mouse model of pancreatic cancer, resulting in a robust inhibition of tumor growth as well as in a significant decrease in the number of metastases.Citation5
Radioactive Listeria (RL) for the Treatment of Pancreatic Cancer
Radioactive Listeriaat (RL) was developed by coupling the radioisotope 188Rhenium (188Re) with Listeriaat by means of anti-Listeria antibodies, a project that we ran in collaboration with the group of Ekaterina Dadachova.Citation5 Mice bearing pancreatic tumors received multiple treatments with low-dose RL, resulting in the nearly complete elimination of metastases and a significant reduction in tumor growth.Citation5 We provided experimental evidence that selectively Listeriaat-infected MDSCs delivered the radioactivity to the primary tumor and metastatic lesions, and that RL infected neoplastic cells. In this setting, cancer cells died upon the delivery of 188Re to their cytoplasm as well as through a “crossfire effect,” i.e., the process whereby 188Re atoms taken up in one cell upon infection by RL also kill non-infected neighboring cells.Citation5,Citation10 The amount of radioactivity (per gram of tissue) accumulated within metastases was 4–5-fold higher than that observed in all other organs, except the liver and kidneys. Extensive pathological studies revealed practically no side effects, not even in normal tissues exposed to comparatively higher amounts of radioactivity such as the liver and kidneys. Presumably, such a good safety profile reflects the fact that highly-proliferating cells, such as malignant cells, are preferentially sensitive to the DNA-damaging effects of radiation. Neither Listeriaat nor radioactivity was detected in non-malignant tissues one week after the last administration of RL. Both 188Re and Listeria-based vaccines have already been tested in cancer patients separately, and only mild side effects were observed.Citation6,Citation9,Citation11 Overall, these observations suggest that RL may constitute a valuable treatment not only for pancreatic cancer, but perhaps also for other tumor types.
Other Microorganisms for Targeting Tumors
Additional studies have shown that MDSCs can be used for the delivery of microorganisms other than Listeria to the TME. For instance, it has been demonstrated that the intravenous administration of oncolytic virus-loaded MDSCs to tumor-bearing mice improves the delivery of vital particles to the TME as well as their local persistence as compared with the systemic injection of naked viruses.Citation12 This results in a significant decrease in tumor burden and increases the survival rate of mice treated with oncolytic virus-loaded MDSCs as compared with animals receiving oncolytic viruses as such. Other groups have demonstrated the potential of bacteria for the selective delivery of anticancer agents to malignant cells.Citation13,Citation14
Other Cellular Vehicles for the Delivery of Anticancer Agents to the TME
MDSCs are not the only type of myeloid cells that home to the TME and hence can be used for the delivery of anticancer agents to malignant cells. For instance, it has been shown that TIE2-expressing monocytes can deliver interferon α to the TME, promoting a near-to-complete inhibition of tumor growth coupled to a significant reduction in the amount of metastases in a xenograft model of human glioma as well as in a transgenic model of mammary adenocarcinoma.Citation15 Mesenchymal stem cells, which normally provide stromal support to malignant lesions, have also been successfully used to deliver anticancer agents to the TME.Citation16 Taken together, these studies (including ours) highlight the great potential of immune cells that naturally home to the TME for selective delivery of anticancer agents.
Summary and Perspectives
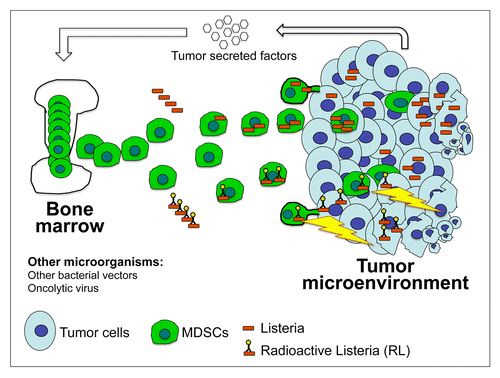
While MDSCs are a major obstacle against the success of anticancer vaccines as they strongly suppress T-cell responses, we demonstrated that a highly attenuated strain of L. monocytogenes (Listeriaat) harnesses MDSCs for reaching the TME, where it infects and kills malignant cells. For the first time, we demonstrated that live Listeriaat bacteria can selectively deliver a radionuclide to the TME with help of MDSCs (). In thus far, MDSCs attack tumor cells like bomb-loaded missiles. Thus, immune cells that naturally home to the TME show great promise for the delivery of anticancer agents to primary neoplastic lesions as well as to metastases.
Figure 1. Myeloid-derived suppressor cells for the delivery of microorganisms or anticancer agents to the tumor microenvironment. Large numbers of myeloid-derived suppressor cells (MDSCs) are released from the bone marrow into the bloodstream of tumor-bearing hosts. MDSCs are attracted to the tumor microenvironment (TME), including primary neoplastic lesions and metastases, by cytokines and other chemoattractants. Upon infection, MDSCs can selectively deliver microorganisms such as an attenuated variant Listeria monocytogenes (Listeria), as such or coupled to a radionuclide (RL), to the TME, where these microorganisms can spread to tumor cells. In thus far, MDSCs attack cancer cells like bomb-loaded missiles. Malignant cells will also be killed through a “crossfire effect,” i.e., the process whereby 188Rhenium (188Re) atoms taken up by one cell upon infection by RL also kill non-infected neighboring cells. With help of MDSCs, RT promotes the accumulation of radionuclides in primary tumors and metastases, promoting a significant inhibition of tumor growth as well as the near-to-complete elimination of metastases in a mouse model of pancreatic cancer. Also oncolytic viruses have been selectively delivered to the TME with the help of MDSCs, resulting in a reduction of tumor burden. Additional bacterial vectors are currently under investigation for the delivery of anticancer agents to the TME. Such novel immunotherapeutic regimens have great potential for the treatment of metastatic tumors.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Chandra D, Gravekamp C. Myeloid-derived suppressor cells: Cellular missiles to target tumors. OncoImmunology 2014; 3:e26967; 10.4161/onci.26967
References
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9:162 - 74; http://dx.doi.org/10.1038/nri2506; PMID: 19197294
- Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182:4499 - 506; http://dx.doi.org/10.4049/jimmunol.0802740; PMID: 19342621
- Liu C, Yu S, Kappes J, Wang J, Grizzle WE, Zinn KR, Zhang HG. Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 2007; 109:4336 - 42; http://dx.doi.org/10.1182/blood-2006-09-046201; PMID: 17244679
- Chandra D, Jahangir A, Quispe-Tintaya W, Einstein MH, Gravekamp C. Myeloid-derived suppressor cells have a central role in attenuated Listeria monocytogenes-based immunotherapy against metastatic breast cancer in young and old mice. Br J Cancer 2013; 108:2281 - 90; http://dx.doi.org/10.1038/bjc.2013.206; PMID: 23640395
- Quispe-Tintaya W, Chandra D, Jahangir A, Harris M, Casadevall A, Dadachova E, Gravekamp C. Nontoxic radioactive Listeria(at) is a highly effective therapy against metastatic pancreatic cancer. Proc Natl Acad Sci U S A 2013; 110:8668 - 73; http://dx.doi.org/10.1073/pnas.1211287110; PMID: 23610422
- Gravekamp C, Paterson Y. Harnessing Listeria monocytogenes to target tumors. Cancer Biol Ther 2010; 9:1 - 9; http://dx.doi.org/10.4161/cbt.9.4.11216; PMID: 20038820
- Kim SH, Castro F, Paterson Y, Gravekamp C. High efficacy of a Listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res 2009; 69:5860 - 6; http://dx.doi.org/10.1158/0008-5472.CAN-08-4855; PMID: 19584282
- Portnoy DA, Auerbuch V, Glomski IJ. The cell biology of Listeria monocytogenes infection: the intersection of bacterial pathogenesis and cell-mediated immunity. J Cell Biol 2002; 158:409 - 14; http://dx.doi.org/10.1083/jcb.200205009; PMID: 12163465
- Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine 2009; 27:3975 - 83; http://dx.doi.org/10.1016/j.vaccine.2009.04.041; PMID: 19389451
- Stritzker J, Szalay AA. Single-agent combinatorial cancer therapy. Proc Natl Acad Sci U S A 2013; 110:8325 - 6; http://dx.doi.org/10.1073/pnas.1305832110; PMID: 23667150
- Klein M, Lotem M, Peretz T, Zwas ST, Mizrachi S, Liberman Y, Chisin R, Schachter J, Ron IG, Iosilevsky G, et al. Safety and efficacy of 188-rhenium-labeled antibody to melanin in patients with metastatic melanoma. J Skin Cancer 2013; 2013:828329; http://dx.doi.org/10.1155/2013/828329; PMID: 23365757
- Eisenstein S, Coakley BA, Briley-Saebo K, Ma G, Chen HM, Meseck M, Ward S, Divino C, Woo S, Chen SH, et al. Myeloid-derived suppressor cells as a vehicle for tumor-specific oncolytic viral therapy. Cancer Res 2013; 73:5003 - 15; http://dx.doi.org/10.1158/0008-5472.CAN-12-1597; PMID: 23536556
- Yu YA, Shabahang S, Timiryasova TM, Zhang Q, Beltz R, Gentschev I, Goebel W, Szalay AA. Visualization of tumors and metastases in live animals with bacteria and vaccinia virus encoding light-emitting proteins. Nat Biotechnol 2004; 22:313 - 20; http://dx.doi.org/10.1038/nbt937; PMID: 14990953
- Patyar S, Joshi R, Byrav DS, Prakash A, Medhi B, Das BK. Bacteria in cancer therapy: a novel experimental strategy. J Biomed Sci 2010; 17:21; http://dx.doi.org/10.1186/1423-0127-17-21; PMID: 20331869
- De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, et al. Tumor-targeted interferon-α delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell 2008; 14:299 - 311; http://dx.doi.org/10.1016/j.ccr.2008.09.004; PMID: 18835032
- Studeny M, Marini FC, Dembinski JL, Zompetta C, Cabreira-Hansen M, Bekele BN, Champlin RE, Andreeff M. Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents. J Natl Cancer Inst 2004; 96:1593 - 603; http://dx.doi.org/10.1093/jnci/djh299; PMID: 15523088