Abstract
Conceptually, the immune system may profoundly influence the efficacy of radiation therapy. Compelling evidence has recently emerged revealing the capacity of local radiation therapy (RT) to induce antitumor immune responses and sparked interest in combining RT with immunotherapy to promote tumor-specific immunity. A Listeria monocytogenes (Lm)-based cancer vaccine engineered to express tumor-associated antigen has been shown to effectively retard tumor growth by cell-mediated immune mechanisms. We hypothesized that combining RT and Lm vaccine will result in synergistic effects that enhance tumor control. Collectively, our data demonstrate that combination therapy significantly delayed B16 melanoma tumor growth by a mechanism partly dependent on CD8+ T cells. Radiotherapy and Lm vaccine each induce different aspects of antitumor immunity, resulting in an overall increase in intratumoral numbers of activated T cells, antigen-specific CD8+ T cells, natural killer (NK) cells and levels of effector molecules, such as interferon γ (IFNγ) and granzyme B. Thus, radiation and Lm vaccine combination therapy is a promising new strategy for the treatment of malignant disease, and further understanding of the mechanisms that underlie efficacy is required to optimize the dosage and schedule for administering the two treatments.
Introduction
The host immune system plays a critical role in influencing cancer disease progression, or potential elimination of tumors. Broadening our understanding of the complex interactions between tumor and immune factors represents the principle basis of oncoimmunological research, a field that has been rapidly growing in the last two decades. Since the host immune system may affect the outcome of treatments, studies have been designed to investigate the effects of conventional cancer therapies on tumor immunity.Citation1 In addition, new strategies to improve conventional cancer therapies are being explored, taking into account the immunological components of tumors.Citation2
In the past, the antitumor activity of local radiation therapy (RT) was attributed predominantly to the ability of ionizing radiation to kill tumor cells by induction of irreparable DNA damage. However, it is now widely recognized that the efficacy of RT is also dependent upon its immunomodulatory effects.Citation3-Citation6 Our lab previously demonstrated that local, single high dose ablative radiation increases the levels of interferon γ (IFNγ) within the tumor, which induces the upregulation of MHC class I molecules on the surface of tumor cells.Citation7 Ablative radiation has also been shown to augment the expression of tumor-associated antigens (TAA)Citation8,Citation9 as a result of mTOR activation that drives increased protein translation.Citation10 These factors potentially lead to better recognition of tumor cells by immune effector cells reactive against TAAs. RT also induces changes in the overall tumor microenvironment. Our lab and others have demonstrated that RT increases the capacity of immune cells to infiltrate tumors.Citation11 Moreover, immunogenic cell death and the release of “danger signals” following RT triggers inflammatory responses.Citation12 These changes in the tumor microenvironment have been suggested to both alleviate immunosuppressive mechanismsCitation13,Citation14 and enhance immunostimulatory processes by altering the type of immune cells recruited to the tumor and influencing their functions.Citation15 Although not completely elucidated, RT has been shown to bring about remodeling of tumor stroma, by modifying extracellular matrix components and tumor vessel endothelial cells.Citation16-Citation18 Additionally, it has been recently reported that local RT can trigger systemic effects,Citation19 such as the reduction of myeloid expansion that is associated with more potent tumor control.Citation20
Despite the generation of antitumor immune responses, RT alone is often insufficient to overcome the immunosuppressive microenvironment of tumors in a sustained manner. Thus, initial reduction in tumor sizes and growth delays typically occur but tumors eventually continue to grow. New knowledge about the immunomodulatory effects of RT have consequently generated interest in combining immunotherapy with RT as an approach to further enhance radiation-induced anti-tumor immunity.Citation21-Citation24 Results of several pre-clinical and clinical studies performed so far have already suggested that the combination of immunotherapy and RT may be a promising approach in cancer treatment. A few examples include cytokine-based therapies such as IL-2,Citation25,Citation26 IL-12Citation27 and Type I interferons,Citation3,Citation28 as well as agents targeting toll-like receptors,Citation29 and treatments aiming to enhance dendritic cell (DC) differentiation, maturation or function.Citation30,Citation31
Listeria monocytogenes (Lm) is an intracellular bacterium that has been developed for use as cancer vaccine vectors by virtue of a natural capacity to elicit potent innate and antigen-specific cellular immune responses. Wild type (WT) Lm, when administered intravenously in mice, is rapidly taken up by antigen-presenting cells, particularly in the spleen and the liver. DCs in the spleen have been shown to play a role in the containment of Lm during early stages of infection, and, to be essential for the priming of adaptive immune responses as demonstrated by the lack of Lm-specific CD8+ T cell activation in DC-depleted CD11c-DTR mice.Citation32 Therefore, Lm is highly effective in stimulating the maturation of DCs, which then efficiently cross-present antigens to activate CD8+ T cells.
Lm expresses internalin (Inl) molecules, InlA and InlB that bind receptors on the surface of host cells, allowing the bacterium to infect non-phagocytic cells.Citation33After successful escape of the bacterium from endocytic or phagocytic vacuoles, Lm replicates in the cytoplasm of the host cell. Deletion of the two virulence factors, ActA (ΔactA) and Inl B (ΔinlB), gives rise to a live-attenuated Lm strain that is still taken up by antigen-presenting cells but has a limited capacity to infect non-phagocytic cells. Further, this ΔactA/ΔinlB Lm mutant strain is defective in cell-to-cell spread after initial infection and replication. This attenuation strategy has been demonstrated to enable rapid clearance of the bacteria in mice (more than 1,000-fold less pathogenic than WT Lm) yet maintaining the generation of a robust immune response.Citation34 Moreover, ΔactA/ΔinlB Lm expressing model antigens have been shown to trigger effective antitumor responses in preclinical models.Citation35 Using the ΔactA/ΔinlB Lm platform, a vaccine strain was engineered by AduroBioTech that expresses a human tumor-associated antigen, mesothelin, commonly overexpressed by tumors such as pancreatic cancer, ovarian cancer, non-small cell lung cancer and mesothelioma. This vaccine, CRS-207, was successfully tested in a Phase I clinical trialCitation36 and has recently completed Phase II clinical trial in combination with low dose cyclophosphamide (CY) and GVAX. GVAX, a vaccine that stimulates a broad antigenic response, is composed of irradiated allogeneic pancreatic cancer cells that have been genetically modified to secrete granulocyte-monocyte colony-stimulating factor. Results from the Phase II trial demonstrated significant extension of overall survival in metastatic pancreatic cancer patients treated with low dose cyclophosphamide (CY)/GVAX plus CRS-207, compared with CY/GVAX alone, reinforcing the benefit of treatments that utilize combinatorial strategies.Citation37
In this study, we used the B16-OVA murine melanoma model to examine the therapeutic potential of combining 15 Gray (Gy) RT with OVA-expressing ΔactA/ΔinlB Lm (Lm-OVA). The combinatorial treatment significantly slowed tumor progression compared with RT or Lm-OVA monotherapies, suggesting a synergism between the two treatments. Tumor control by Lm-OVA vaccine was completely abolished in mice depleted of CD8+ T cells, whereas growth delay following RT was only partially dependent on CD8+ T cells. Our initial investigation of possible mechanisms underlying the enhanced efficacy of combination therapy suggests that Lm-OVA treatment increases the number of total CD8+ T cells, antigen-specific CD8+ T cells and CD4+ T cells. Moreover, expression of the activation marker, CD69 on both CD4+ and CD8+ T cells was upregulated in tumors that were treated with 15 Gy RT, suggesting that these cells had acquired a more activated phenotype. Although an additive increase was only observed in the number of tumor-infiltrating NK cells, Lm-OVA vaccine and RT seem to activate different components of the immune system that when combined, contribute to enhanced antitumor potency.
Taken together, our data suggest that Lm vaccine combined with RT can elicit better tumor control, at least partly as each treatment evokes distinct immune responses that complement one another in a synergistic manner that leads to tumor growth suppression.
Results
Lm-OVA vaccine and radiation combination therapy retard tumor growth better than either treatment alone
To test our hypothesis that combinatorial therapy is more effective at delaying tumor growth than Lm-OVA vaccine or RT monotherapy, we injected B16-OVA intramuscular (i.m.) into the thigh on day 0 and monitored tumor growth over time. Mice were given 15 Gy single dose radiation on day 7, or Lm-OVA on day 8, or both. In order to control for the non-OVA-specific effects on tumor growth resulting from attenuated Lm infection, additional mice were treated with the same strain of Lm that does not express OVA (Lm-Ctrl). Tumors in mice that were given Lm-OVA alone continued to grow for about 5 d before starting to decrease in size, whereas administration of Lm-Ctrl did not have an effect on tumor growth (), similar to mice given only BSS (data not shown). However, at later time points (mean = 27 d), mean thigh diameters of mice treated with only Lm-OVA eventually exceeded 12 mm and the mice had to be euthanized.
Figure 1. Radiation therapy combined with Listeria monocytogenes-based vaccine delays B16 melanoma growth further than either monotherapy. (A-F) To assay the efficacy of radiation therapy (RT) and Listeria monocytogenes (Lm) vaccine combinatorial therapy, C57Bl/6 recipient mice were implanted with 1 x 105 OVA-expressing B16 cells injected i.m. into the left leg of each mouse. On day 7, mice were left untreated (A and B) or treated with 15 Gy radiation on the tumor-bearing leg (C and D) and on day 8, mice were injected with either Lm-OVA (red lines), or Ctrl-Lm (blue lines). For depletion of CD8+ T cells (B and D), 200 μg anti-CD8 antibody was administered i.p. on days 6, 8, 10 and 12. Tumor growth was monitored by measuring the thigh diameter every other day. Dashed lines indicate the approximate window of time within which mice treated with radiation had to be euathanized. E. Extension of growth curves from (C). F. Thigh diameters after tumor rechallenge on the opposite leg among recipient surviving longer than 60 d. Data shown are combined results of 2 independent experiments (n = 6 to 9).
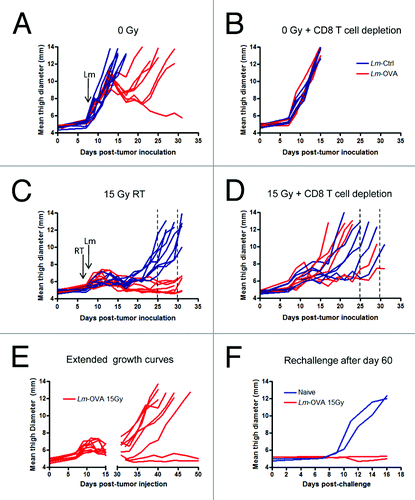
To determine the role of CD8+ T cells in Lm-OVA alone therapy, mice were given anti-CD8 antibody intravenously starting on day 6, a time point when palpable tumors were established and before the initiation of treatment. Depletion of host CD8+ T cells resulted in complete abrogation of the reduction in tumor size by Lm-OVA treatment (). This suggests that the effect of Lm-OVA on tumor growth is highly dependent on CD8+ T cells. We next examined the combined effect of both RT and Lm vaccine. Although RT plus Lm-Ctrl significantly delayed growth of B16 melanoma, reaching end-point sizes between day 25 and 30, RT plus Lm-OVA combination therapy was superior in comparison (). Among mice that lack CD8+ T cells and were treated with 15 Gy RT plus Lm-Ctrl, there was a trend (although not statistically significant) toward slightly faster tumor growth than immunocompetent mice given the same treatment. Therefore, the ability of irradiation to control tumor growth is not completely dependent on CD8+ T cells. With RT plus Lm-OVA, loss of the therapeutic effect was also observed in mice given CD8 depleting antibody (). Significantly larger tumors were observed in CD8-depleted mice treated with the combinatorial therapy compared with non-depleted mice starting at day 17, and continuing until day 21 when tumors began to reach sizes that required the mice to be euthanized. However, the extent to which tumor growth delay was compromised by CD8 depletion was variable. Thus, combination therapy requires CD8+ T cells to attain maximal therapeutic potential, but CD8+ T cells are most likely not the only effector cells that contribute to the dramatic delay in tumor growth.
Interestingly, after a long period with no signs of progression, rapid tumor outgrowth occurred in most of the mice that were given RT plus Lm-OVA combination therapy (). However, the other 20% of mice in this treatment group had no measureable tumors even at day 60. These mice were subsequently rechallenged with B16-OVA injected i.m. into the opposite leg. As expected, these mice were protected from tumor growth after tumor challenge at a distal site without additional treatment (). These results suggest that high-dose radiation and Lm-OVA treatments can synergize to bring about improved and durable antitumor responses in a manner at least partially dependent on CD8+ T cells.
Radiation therapy and Lm-OVA vaccine alter the number and activation status of tumor-infiltrating T cells
To understand the possible mechanisms driving improved tumor control following combination therapy, we next evaluated how the tumor environment was altered in response to the different treatments. B16-OVA cells were injected on day 0, treated with irradiation and Lm-OVA (as described above) and on day 13, tumors were excised for cytofluorimetric analysis of tumor-infiltrating lymphocytes. At this time point, the relative number of intratumoral CD8+ T cells in mice administered Lm-Ctrl was not increased by RT (). Treatment with Lm-OVA alone slightly increased the number of intratumoral CD8+ T cells compared with mice that were given Lm-Ctrl, suggesting that the antitumor immune response generated by the vaccine was dependent on antigen-specificity. Importantly, the increase in number of tumor-infiltrating CD8+ T cells was significantly higher when RT and Lm-OVA were administered in combination as compared with mice treated with RT plus Lm-Ctrl (). Surprisingly, the number of CD8+ T cells in tumors treated with combination therapy was not significantly higher than mice receiving Lm-OVA monotherapy, despite a marked delay in tumor growth in mice treated with RT and Lm-OVA combinatorial therapy relative to those given Lm-OVA alone (). Upon examining the number of tumor-infiltrating CD4+ T cells, a similar trend was observed (). Further phenotypic analysis by flow cytometry revealed that in mice receiving any of the treatments, the majority of tumor-infiltrating T cells (on average more than 80%) were CD44high CD62Llow, hence of the effector cell phenotype (data not shown).
Figure 2. Combination therapy affects the number and activation state of CD4+ and CD8+ tumor-infiltrating T cells. (A-D) To assay the effects of radiation therapy and Listeria monocytogenes (Lm) vaccine combinatorial therapy on tumor-infiltrating lymphocytes, 1 x 105 OVA-expressing B16 cells were injected intramuscularly into the thigh and mice were treated with 0 Gy or 15 Gy on day 7, and Ctrl-Lm or Lm-OVA on day 8. Tumors were excised on day 13, weighed and processed into single cell suspensions by collagenase treatment. (A-B) Tumor-infiltrating lymphocytes were detected by staining with fluorescence-conjugated antibodies for an hour followed by cytofluorimetric analysis. T cells were identified by sequential gating for cells that were CD45+ CD3+ and either CD8+ or CD4+. The number of tumor-infiltrating CD8+ (A) and CD4+ (B) T cells were expressed as cells per mg of each tumor. (C-D) The expression levels of the activation marker CD69 on CD8+ (C) and CD4+ (D) T cells were similarly analyzed by immunofluorescence staining and flow cytometry. Data are shown as mean fluorescence intensity (MFI) values. Statistical significance was evaluated using one-way ANOVA; comparisons that are not labeled with a P value are not significantly different. Data shown are representative of two independent experiments.
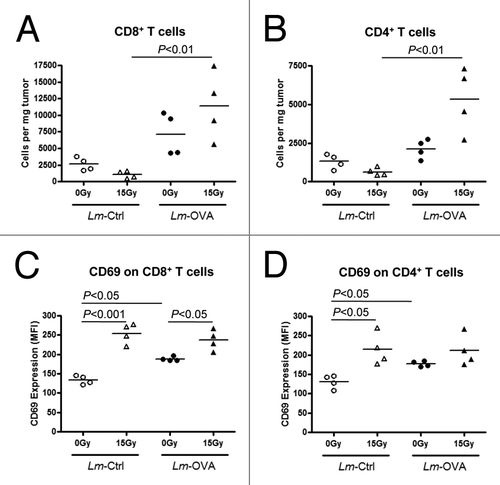
Besides changes to the number of T cells within the tumor, it is also possible that the treatments alter the activation status of these effector cells. Indeed, the cell surface expression of CD69, an early activation marker, on CD8+ T cells () and CD4+ T cells () was increased following 15 Gy RT both in mice that were given Lm-Ctrl, as well as in mice administered Lm-OVA. However, although treatment with Lm-OVA alone increased the expression of CD69 on CD8+ and CD4+ T cells compared with that of Lm-Ctrl, the vaccine did not seem to further increase the activation status of T cells in mice that were treated with the combination of RT and Lm-OVA relative to RT only (15 Gy plus Lm-Ctrl). Thus far, our data suggest that when administered in combination, RT and Lm-OVA increase the recruitment of CD8+ and CD4+ T cells into the tumor, and these tumor-infiltrating CD8+ and CD4+ T cells are more strongly activated, as assessed by CD69 expression.
Lm-OVA significantly increases antigen-specific T cells that infiltrate the tumor
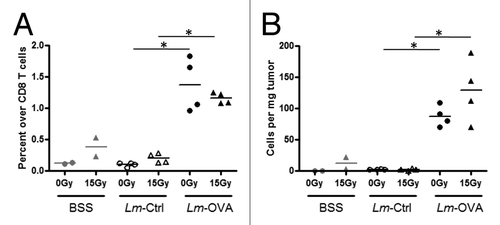
Effective antitumor immune responses are dependent on cytotoxic CD8+ T cells that can specifically recognize TAAs, a process required for targeted killing of tumor cells. Thus, the number of antigen-specific CD8+ T cells within the tumor should naturally contribute to the potency of an antitumor immune response. To enumerate antigen-specific CD8+ T cells in B16-OVA tumors, MHC class I-restricted OVA-dextramers were used to identify SIINFEKL-specific CD8+ T cells. To control for OVA-specific CD8+ T cells generated by the inoculation of B16-OVA cells itself, mice were inoculated with parental B16 cells and treated with or without radiation on day 7, and balanced saline solution (BSS) on day 8. These mice provided the baseline numbers and percentages of OVA-specific CD8+ T cells in the absence of any exogenous OVA antigen and Lm infection. Neither Lm-Ctrl only nor Lm-Ctrl plus 15 Gy RT significantly altered the percentage () or number () of OVA-specific CD8+ T cells compared with the parental B16 controls. On the other hand, Lm-OVA alone induced a marked increase in the percentage () and number () of OVA-specific CD8+ T cells, increasing by ~100 cells per mg tumor tissue. The addition of RT did not result in any further significant increase. Taken together, Lm-OVA is a potent stimulus that brings about an increase in the number of total T cells as well as antigen-specific CD8+ T cells. Although RT did not seem to enhance this response, it instead provided an activation signal that increased CD69 expression on almost all CD4+ and CD8+ T cells within the tumor. These two factors together, may potentiate the delay in tumor progression in mice that received the RT plus Lm-OVA combinatorial treatment.
Figure 3.Lm-OVA vaccine therapy increased the frequency and number of OVA-specific CD8+ T cells within the tumor. (A-B) To assay the effects of radiation therapy and Listeria monocytogenes (Lm) vaccine combinatorial therapy on tumor antigen-specific T cells, 1 x 105 B16-OVA cells were injected intramuscularly into the thigh and mice were treated with 0 Gy or 15 Gy on day 7, and Ctrl-Lm or Lm-OVA on day 8. Tumors were excised on day 13, weighed and processed into single cell suspensions by collagenase treatment. Cells were stained with fluorescence-conjugated antibodies for an hour and followed by cytofluorimetric analysis. To detect antigen-specific CD8+ T cells, PE-conjugated H-2Kb/SIINFEKL dextramers (Immundex) were used to label cells prior to staining with anti-CD45, -CD3 and -CD8 antibodies. OVA-dextramer+ cells were expressed in terms of (A) percentage over total CD8+ T cells (B) relative number of cells in 1 mg of each tumor. Data shown are representative of two independent experiments. In each experiment, n = 2 for balanced saline solution (BSS) controls, and n = 4 for mice that were treated with Lm-Ctrl or Lm-OVA. Statistical significance was evaluated using one-way ANOVA.
The number of tumor-infiltrating NK cells is increased following combinatorial therapy
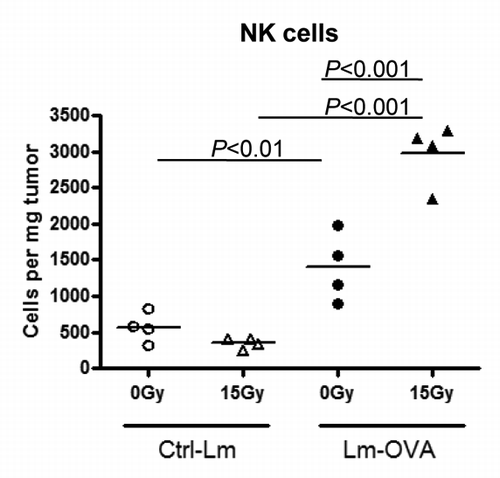
We had previously observed that depletion of CD8+ T cells using anti-CD8 antibody only partially abrogated tumor growth delay in response to RT and Lm-OVA combinatorial therapy in about 33% of mice (). Therefore, other effector cells and molecules could be contributing to tumor control in response to combinatorial therapy. NK cells have been shown to have the ability to kill tumor cells in a non-MHC-restricted, non-antigen-specific manner. As shown in , for mice that were treated with Lm-Ctrl, 15 Gy did not exhibit increased NK cells within the tumor. On the other hand, Lm-OVA vaccine monotherapy induced recruitment and significant accumulation of more NK cells in the tumor, and the number of NK cells was further elevated following RT plus Lm-OVA combinatorial therapy to approximately ~2X that of Lm-OVA only and ~10X over RT only (P value < 0.001; ). In conclusion, besides CD8+ T cells, NK cells may also play a role in the combinatorial therapy-mediated antitumor response that brings about prolonged maintenance of tumors in a progression-free state.
Figure 4. Combination therapy increased the number of NK cells within the tumor. To assay the effects of radiation therapy and Listeria monocytogenes (Lm) vaccine combinatorial therapy on natural killer (NK) cells, 1 x 105 B16-OVA cells were injected intramuscularly into the thigh and mice were treated on the tumor-bearing leg with 0 Gy or 15 Gy on day 7, and Ctrl-Lm or Lm-OVA on day 8 (n = 4 per group). Tumors were excised on day 13, weighed and processed into single cell suspensions by collagenase treatment. Cells were stained with fluorescence-conjugated antibodies for an hour followed by cytofluorimetric analysis. NK cells were gated based on the markers CD45+ CD3- NK1.1+. Data shown are representative of 2 independent experiments. Statistical significance was evaluated using one-way ANOVA.
Effector molecules are increased by Lm vaccine and irradiation in a complementary manner
To gain insight on the functionality of effector cells within the tumor, we investigated the levels of 2 molecules in the tumor microenvironment that may be indicative of effector cell function. First, IFNγ levels in whole tumor lysates were detected by ELISA. Lm-OVA vaccine alone was sufficient to significantly increase the levels of IFNγ within the tumor (P value < 0.001; ). Congruent with CD8+ T cell numbers, combinatorial therapy did not further increase the levels of IFNγ compared with that of Lm-OVA alone.
Figure 5. IntratumoralIFNγ protein levels and granzyme B mRNA levels were not upregulated in additive or synergistic manner in combinatorial treatment. (A-B) B16-OVA cells were injected intramuscularly into the thigh. Recipient mice were treated locally with 0 Gy or 15 Gy on day 7, and Ctrl-Lm or Lm-OVA on day 8 (n = 3 per group). Tumors were excised on day 13 as before and analyzed for effector molecule protein (A) and RNA (B) expression levels. (A) Supernatants of tumor homogenates were used to determine interferon γ (IFNγ) levels within the tumor by IFNγ-specific ELISA. The concentration of IFNγ in each sample was normalized to the total protein concentration in the sample. Statistical significance was evaluated using one-way ANOVA (n = 6–7; combined data from 2 experiments). (B) Quantitative reverse transcription PCR (qRT-PCR) analysis of transcripts encoding IFNγ (left) and Granzyme B (right). Total mRNA in each tumor homogenate was isolated and qRT-PCR was performed. Granzyme B transcript levels in each sample was normalized by GAPDH levels and expressed as fold increases over balances saline solution (BSS) + 0 Gy control. Data shown are representative of 2 independent experiments.
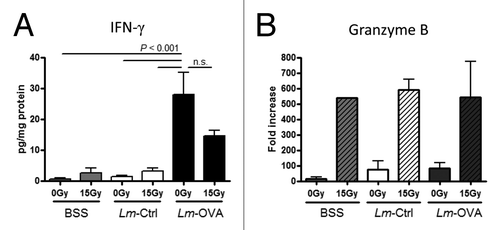
Next, we measured the transcript levels of the granzyme B gene (GZMB) in whole tumor lysates. Activated CD8+ T cells and NK cells can upregulate the expression of cytotoxic molecules including granzyme B. Together with perforin which form pores in the target cell membrane, granzyme B released by effector cells has been shown to induce apoptosis of tumor cells.Citation38 Strikingly, RT was the only therapy that resulted in an induction in the relative mRNA levels of GZMB, and these levels of induction are consistent among mice given BSS, Lm-Ctrl or Lm-OVA (). In support, intracellular granzyme B staining and analysis by flow cytometry on day 13 revealed that tumor-associated CD4+ T cells, CD8+ T cells as well as NK cells have higher expression levels of granzyme B protein following RT (data not shown). In conclusion, Lm-OVA induces production of more IFNγ in the tumor whereas RT upregulates granzyme B as well as IFNγ. Taken together with our data demonstrating an increase in the number of T cells following the various treatments ( and ), these results suggest that radiation and Lm-OVA have several distinct effects on antitumor immune response. Therefore, in combination RT and Lm-OVA vaccine are complementary treatments, inducing a more comprehensive anticancer immunological response.
Discussion
Recent studies suggest that radiation therapy is more efficacious in treating cancer when used in combination with immunotherapy. In this study, we show that the delay in tumor progression (i.e., growth) achieved by combining Lm-OVA vaccine with radiation therapy is superior to the administration of either treatment as a monotherapy. The small proportion of mice that underwent tumor regression were found to be protected against tumor rechallenge at a later time point, suggesting the formation of immunological memory induced by the combinatorial regimen.
To our knowledge, our report is only the second preclinical study investigating the therapeutic potential of combining RT and a Lm-based vaccine.Citation39 By focusing on evaluating the local tumor microenvironment, our data extend the earlier finding by Hannan et al. that a similar combinatorial strategy results in additive effects on systemic immune responses.Citation39
Our results suggest that RT plus Lm-OVA enhances immune-mediated antitumor mechanisms within the tumor microenvironment. In addition to increased numbers of tumor-infiltrating CD8+ T cells, these cells have increased expression of CD69, which is indicative of a higher activation status. Moreover, the number of tumor-infiltrating NK cells are significantly increased following administration of RT plus Lm-OVA. Strong evidence from previous studies support the concept that CD8+ T cells recognize MHC class I-positive target cells, whereas NK cells mediate killing of cells that downregulate MHC class I molecules, a process described by many as “missing-self” recognition.Citation40-Citation42 Therefore, the dual increase in tumor-infiltration by CD8+ T cells and NK cells observed following RT plus Lm-OVA may be an important benefit of the combination treatment, an observation warranting future investigations.
IFNγ and granzyme B, are effector molecules secreted by both T cells and NK cells. In B16 tumors, IFNγ is mainly produced by CD4+ T cells, CD8+ T cells and NK cells (data not shown). The marked increases in numbers of tumor-infiltrating CD8+ T cells and NK cells in mice treated with Lm-OVA or RT plus Lm-OVA correspond with the significant elevation in intratumoral levels of IFNγ observed in response to the combination therapy. Although intratumoral levels of IFNγ were similar in mice given Lm-OVA and RT plus Lm-OVA combination therapy, this ELISA-based experiment only detects IFNγ present in the tissue at a particular time point and does not account for cytokine that has been secreted but consumed. Therefore, it is possible that the actual levels of IFNγ produced by effector cells may be underestimated. For analysis of granzyme B, intratumoral GZMB transcript levels were markedly increased in mice that received RT regardless of whether Lm-OVA was administered.
The results of our study are summarized in . Overall, we conclude that maximal therapeutic outcome was achieved when both the quantity and quality of immune effector cells present at the tumor site were enhanced. For example, the number of CD8+ T cells, along with the quality, as assessed by CD69 and granzyme B expression, is highest in mice treated with RT plus Lm-OVA. Using the number of days to tumor end point (12 mm in mean size) as a measure, RT plus Lm-OVA resulted in the longest tumor control compared with the other treatments. Therefore, it is the summation of different effector mechanisms elicited by Lm vaccine and RT that resulted in better tumor control rather than an enhancement in one particular aspect of the antitumor immune response. In the future, further understanding of these mechanisms will be critical for treatment optimization in the clinical setting.
Table 1.Lm vaccine and radiation therapy have complementary effects in activating anti-tumor immunity
Exploring variations on dosing and scheduling of the treatments may be essential to improve the capacity of the combination therapy to eliminate tumor progression in patients. In our experiments in mice, radiation treatment was administered one day prior to a single dose of Lm-OVA vaccine. The rationale behind our experimental design was that RT has the potential to reduce tumor burden, which may allow for better intervention by subsequent vaccine treatment. Moreover, initiation of antitumor responses by RT, a process that has been proposed to “convert” irradiated tumor into a vaccine in situ,Citation43 is likely to be boosted by the strong immunostimulatory effects of Lm-based vaccine. Furthermore, RT has been shown to alter the tumor landscape, resulting in a more conducive microenvironment for immune cell infiltration and activation. Administering RT before Lm-based vaccine may favor rapid recruitment of Lm-generated OVA-specific T cells to the tumor site once they leave the spleen or tumor-draining lymph node. Although, 15 Gy RT on day 7 followed by Lm-OVA administration on day 8 efficaciously prolonged the phase of stable disease and in a few cases, resulted in tumor regression, it is possible that additional doses of vaccine may prevent the loss of tumor control and bring about tumor regression. In the CRS-207 vaccine Phase I clinical trial, multiple inoculations of the ΔactA/ΔinlB Lm-based cancer vaccine were well tolerated without accumulating toxicities.Citation36 Thus, it is anticipated that the combined therapy of radiation and Lm-based vaccination, potentially involving multiple vaccine doses, will be feasible and safe.
The type of RT strategy used in our study is single, high dose local radiation, also known as ablative or stereotactic body radiation therapy. Although fractionated, low dose RT (conventional RT) is traditionally used in the clinic to treat cancer patients, the use of ablative radiation therapy is increasingly common in certain cancers, due to recent studies suggesting ablative strategies can be more beneficial.Citation18,Citation44,Citation45 In our study, RT + Lm-OVA efficiently induced the recruitment of CD4+ T cells, CD8+ T cells and NK cells into the tumor. In the context of an antitumor immune response, a single dose of radiation may be more effective since a fractionated regime of RT is likely to deplete these radio-sensitive immune cells that infiltrate the tumor, which is counterproductive when attempting to enhance antitumor immunity. In addition to inducing an immune response, high dose RT is also likely to work synergistically with Lm-OVA vaccine by directly killing tumor cells, thus debulking the tumor mass. RT has also been shown to destroy tumor vessels, reducing the supply of oxygen and nutrients to tumor cells. Therefore, besides the requirement for CD8+ T cells and other immune components, we believe that high-dose RT enhances Lm-based vaccine by multiple mechanisms.
In conclusion, our results reveal the promising therapeutic potential of combining RT and ΔactA/ΔinlB Lm-based cancer vaccine and elucidate the chief mechanisms contributing to the synergy of co-administering these treatments. We expect that this therapeutic regimen can be applicable to other types of cancer that have been shown to respond to radiation. Further pre-clinical and clinical investigations will provide the necessary data to optimize the timing and dosing of each treatment for further improvement, and develop the clinical applicability of this combinatorial approach.
Materials and Methods
Mice and cell lines
C57BL/6J wild-type female mice were purchased from The Jackson Laboratory (Bar Harbor, ME) at 6–8 wk of age. Mice were maintained at the animal facility in University of Rochester in accordance with guidelines for the humane treatment of animals, and experiments were performed using protocols approved by the University Committee on Animal Resources. B16-F0, a cell line derived from a spontaneous melanoma of a C57BL/6 mouse,Citation46 was obtained from the American Type Culture Collection (CRL 6322). cDNA encoding chicken ovalbumin (OVA) was transfected into B16-F0 by lipofection and a single clone of B16-OVA was selected following cloning by limiting dilution.Citation47 Cells were maintained in culture as previously described,Citation48 with the addition of selection media, G418, to B16-OVA cells at 400 μg/mL.
Tumor growth and measurement
On the day of tumor cell inoculation, single cell suspensions were prepared from B16-OVA cells in culture. Harvested cells were washed with balanced saline solution (BSS) and injected intramuscularly (i.m.) into the lower left thigh of mice at 1 x 105 cells per mouse. Tumor growth was monitored by measuring thigh diameters of mice in 2 dimensions using digital calipers, and the values were expressed as mean thigh diameter, as described before.Citation48 Mice were euthanized when mean thigh diameters exceeded 12mm, and the number of days since tumor inoculation was recorded as days to tumor end-point size.
Local single high-dose radiation treatment
Mice were treated on the tumor-bearing leg with a single dose of 15 Gy radiation, 7 d post-tumor inoculation, a time point corresponding to an average mean thigh diameter of 5.34 mm (n = 24). Irradiation was performed on non-anesthetized mice that were restrained as previously described, using a 137Cs source that operates at a dose rate of about 1.90 Gy/min.Citation11
Preparation and injection of Lm vaccine
All strains of Lm used were obtained from Aduro Biotech. ΔactA/ΔinlB Lm was derived from the wild-type strain 10403S, and an antigen expression cassette for OVA was inserted, as previously described.Citation34 On day 8 (one day post-RT) mice were injected intravenously (i.v.) with 0.1 LD50 of ΔactA/ΔinlB Lm that expresses OVA (Lm-OVA) or ΔactA/ΔinlB Lm that does not express a transgene (Lm-Ctrl).
Depletion of CD8+ T cells in mice
To deplete CD8+ T cells in mice with established tumors, 200 μg anti-CD8α antibody (clone 53–6.7) was injected intraperitoneally (i.p.), starting one day before radiation treatment (day 6), and every other day, for a total of four doses. This protocol was previously optimized by our lab to obtain consistent depletion of CD8+ T cells in mice that is sustainable for more than 1 wk.
Tumor dissociation, immunofluorescence staining, and flow cytometry
Mice were sacrificed and tumors were excised day 13 post-tumor inoculation. This time point was chosen for tumor microenvironment comparisons because immune responses induced by both radiation and Lm vaccine therapies were ongoing. Moreover, after day 13, tumors from mice treated with RT combined with Lm-OVA were too small in size for assessment. After removal, tumors were mechanically cut into smaller pieces, digested with collagenase as described previouslyCitation7 and single cell suspensions were incubated with LIVE/DEAD® aqua dead cell stain (Invitrogen, Grand Island, NY) according to manufacturer's protocol prior to surface staining. Fluorescence-conjugated antibodies targeting mouse CD3 (145–2C11), CD4 (GK1.5), CD8 (Ly-2, 53–6.7), CD16/CD32 (Fc Block) (2.4G2), CD45 (30-F11), NK1.1 (PK136), CD69 (H1.2F3), and isotype controls were from BD Biosciences and eBioscience. To identify OVA-specific CD8+ T cells, Phycoerythrin-labeled MHC Dextramer® (Immudex, Copenhagen, Denmark) carrying SIINFEKL-H-2Kb complexes was incubated with cell suspensions according to manufacturer's protocol after LIVE/DEAD® staining and before labeling of cell surface markers. Following immunofluorescence staining, cytofluorimetric analysis was performed on a BD FACSCantoTM II flow cytometer and data analyzed using FlowJo software (Tree Star, Inc). OVA-specific CD8+ T cells were expressed as a percentage over total live CD8+ T cells in the tumor.
ELISA
Tumor pieces were snap-frozen in lysis buffer containing 50mM Tris (pH7.4), 300mM NaCl, 10% glycerol, 3mM EDTA, 1mM MgCl2, 20mM β-glycerophosphate, 25mM NaF, 1% Triton X-100 and protease inhibitors (BioVision).Citation49 Samples were thawed on ice, processed using a motorized tissue homogenizer (PRO Scientific), and cell debris was pelleted by centrifugation. Total protein concentration in the supernatant collected from each sample was determined by BCA assay (Thermo Fisher Scientific) and IFNγ levels were assessed using ELISA kit from eBioscience. IFNγ concentration was calculated by taking the ELISA value of each sample normalized to the total protein level in the sample.
Real-time quantitative PCR
Tumors were excised and sample pieces were snap-frozen in buffer RLT (QIAGEN). RNA was isolated using the RNeasy kit (QIAGEN) from tumor homogenates and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed, as described previously.Citation50The intron-spanning forward and reverse primers used were designed in-house and obtained from Eurofins MWG Operon. Sequences of primers used are as follows. For GAPDH: Forward, 5′-CATTGCTCTC AATGACAACT-3′, Reverse, 5′-GGGTTTCTTA CTCCTTGGAG-3′. For Granzyme B: Forward, 5′-GGAAGATGAA GATCCTCCTG-3′, Reverse, 5′-ATCGAAAGTA AGGCCATGTA-3′. All samples were normalized based on GAPDH threshold cycle values and fold changes over a randomly chosen sample in the control group were calculated using 2−□ΔΔCt formulae by comparative Ct method.
Statistical analysis
All statistical analyses were performed using the Prism software package v.4.0 (GraphPad Software, Inc., La Jolla, CA). Unless indicated otherwise, multiple group comparisons were performed using one-way ANOVA and P values were adjusted using Bonferroni correction.
| Abbreviations: | ||
| BSS | = | balanced saline solution |
| Ctrl | = | control |
| CY | = | cyclophosphamide |
| GZMB | = | granzyme B gene |
| i.m. | = | intramuscular |
| i.p. | = | intraperitoneal |
| i.v. | = | intravenous |
| IFNγ | = | interferon γ |
| Inl | = | internalin |
| Lm | = | Listeria monocytogenes |
| NK | = | natural killer |
| OVA | = | chicken ovalbumin |
| RT | = | radiation therapy, TAA, tumor-associated antigen |
Disclosure of Potential Conflicts of Interest
D.G.B. is an employee and shareholder in AduroBioTech, Inc. The other authors have no potential conflicts of interest.
Acknowledgments
The authors thank Dr Stephen T Haley and Immudex USA, LLC for their generous support in providing MHC Dextramers® required for this study. This project was financially supported by the National Institutes of Health grant CA 28332.
References
- Hodge JW, Garnett CT, Farsaci B, Palena C, Tsang KY, Ferrone S, Gameiro SR. Chemotherapy-induced immunogenic modulation of tumor cells enhances killing by cytotoxic T lymphocytes and is distinct from immunogenic cell death. Int J Cancer 2013; 133:624 - 36; http://dx.doi.org/10.1002/ijc.28070; PMID: 23364915
- Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G. Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 2013; 39:74 - 88; http://dx.doi.org/10.1016/j.immuni.2013.06.014; PMID: 23890065
- Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, Beckett M, Sharma R, Chin R, Tu T, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114:589 - 95; http://dx.doi.org/10.1182/blood-2009-02-206870; PMID: 19349616
- Shiao SL, Coussens LM. The tumor-immune microenvironment and response to radiation therapy. J Mammary Gland Biol Neoplasia 2010; 15:411 - 21; http://dx.doi.org/10.1007/s10911-010-9194-9; PMID: 21161342
- Takeshima T, Chamoto K, Wakita D, Ohkuri T, Togashi Y, Shirato H, Kitamura H, Nishimura T. Local radiation therapy inhibits tumor growth through the generation of tumor-specific CTL: its potentiation by combination with Th1 cell therapy. Cancer Res 2010; 70:2697 - 706; http://dx.doi.org/10.1158/0008-5472.CAN-09-2982; PMID: 20215523
- Perez CA, Fu A, Onishko H, Hallahan DE, Geng L. Radiation induces an antitumour immune response to mouse melanoma. Int J RadiatBiol 2009; 85:1126 - 36; http://dx.doi.org/10.3109/09553000903242099; PMID: 19995238
- Lugade AA, Sorensen EW, Gerber SA, Moran JP, Frelinger JG, Lord EM. Radiation-induced IFN-gamma production within the tumor microenvironment influences antitumor immunity. J Immunol 2008; 180:3132 - 9; http://dx.doi.org/10.4049/jimmunol.180.5.3132; PMID: 18292536
- Santin AD, Hermonat PL, Ravaggi A, Chiriva-Internati M, Pecorelli S, Parham GP. Radiation-enhanced expression of E6/E7 transforming oncogenes of human papillomavirus-16 in human cervical carcinoma. Cancer 1998; 83:2346 - 52; http://dx.doi.org/10.1002/(SICI)1097-0142(19981201)83:11<2346::AID-CNCR14>3.0.CO;2-G; PMID: 9840534
- Sharma A, Bode B, Wenger RH, Lehmann K, Sartori AA, Moch H, Knuth A, Boehmer Lv, Broek Mv. γ-Radiation promotes immunological recognition of cancer cells through increased expression of cancer-testis antigens in vitro and in vivo. PLoS One 2011; 6:e28217; http://dx.doi.org/10.1371/journal.pone.0028217; PMID: 22140550
- Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, Camphausen K, Luiten RM, de Ru AH, Neijssen J, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203:1259 - 71; http://dx.doi.org/10.1084/jem.20052494; PMID: 16636135
- Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol 2005; 174:7516 - 23; http://dx.doi.org/10.4049/jimmunol.174.12.7516; PMID: 15944250
- Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 2007; 13:1050 - 9; http://dx.doi.org/10.1038/nm1622; PMID: 17704786
- Wei S, Egenti MU, Teitz-Tennenbaum S, Zou W, Chang AE. Effects of tumor irradiation on host T-regulatory cells and systemic immunity in the context of adoptive T-cell therapy in mice. J Immunother 2013; 36:124 - 32; http://dx.doi.org/10.1097/CJI.0b013e31828298e6; PMID: 23377667
- Billiard F, Buard V, Benderitter M, Linard C. Abdominal γ-radiation induces an accumulation of function-impaired regulatory T cells in the small intestine. Int J RadiatOncolBiolPhys 2011; 80:869 - 76; http://dx.doi.org/10.1016/j.ijrobp.2010.12.041; PMID: 21345609
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low-dose irradiation programs macrophage differentiation to an iNOS⁺/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell 2013; 24:589 - 602; http://dx.doi.org/10.1016/j.ccr.2013.09.014; PMID: 24209604
- Fenton BM, Lord EM, Paoni SF. Effects of radiation on tumor intravascular oxygenation, vascular configuration, development of hypoxia, and clonogenic survival. Radiat Res 2001; 155:360 - 8; http://dx.doi.org/10.1667/0033-7587(2001)155[0360:EOROTI]2.0.CO;2; PMID: 11175672
- Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment - tumorigenesis and therapy. Nat Rev Cancer 2005; 5:867 - 75; http://dx.doi.org/10.1038/nrc1735; PMID: 16327765
- Park HJ, Griffin RJ, Hui S, Levitt SH, Song CW. Radiation-induced vascular damage in tumors: implications of vascular damage in ablative hypofractionated radiotherapy (SBRT and SRS). Radiat Res 2012; 177:311 - 27; http://dx.doi.org/10.1667/RR2773.1; PMID: 22229487
- Formenti SC, Demaria S. Systemic effects of local radiotherapy. Lancet Oncol 2009; 10:718 - 26; http://dx.doi.org/10.1016/S1470-2045(09)70082-8; PMID: 19573801
- Crittenden MR, Savage T, Cottam B, Bahjat KS, Redmond WL, Bambina S, Kasiewicz M, Newell P, Jackson AM, Gough MJ. The peripheral myeloid expansion driven by murine cancer progression is reversed by radiation therapy of the tumor. PLoS One 2013; 8:e69527; http://dx.doi.org/10.1371/journal.pone.0069527; PMID: 23936036
- Seung SK, Curti B, Crittenden M, Urba W. Radiation and immunotherapy: Renewed allies in the war on cancer. Oncoimmunology 2012; 1:1645 - 7; http://dx.doi.org/10.4161/onci.21746; PMID: 23264923
- Burnette B, Fu YX, Weichselbaum RR. The confluence of radiotherapy and immunotherapy. Front Oncol 2012; 2:143; http://dx.doi.org/10.3389/fonc.2012.00143; PMID: 23087904
- Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J RadiatOncolBiolPhys 2005; 63:655 - 66; http://dx.doi.org/10.1016/j.ijrobp.2005.06.032; PMID: 16199306
- Kalbasi A, June CH, Haas N, Vapiwala N. Radiation and immunotherapy: a synergistic combination. J Clin Invest 2013; 123:2756 - 63; http://dx.doi.org/10.1172/JCI69219; PMID: 23863633
- Seung SK, Curti BD, Crittenden M, Walker E, Coffey T, Siebert JC, Miller W, Payne R, Glenn L, Bageac A, et al. Phase 1 study of stereotactic body radiotherapy and interleukin-2--tumor and immunological responses. SciTransl Med 2012; 4:37ra74; http://dx.doi.org/10.1126/scitranslmed.3003649; PMID: 22674552
- Younes E, Haas GP, Dezso B, Ali E, Maughan RL, Kukuruga MA, Montecillo E, Pontes JE, Hillman GG. Local tumor irradiation augments the response to IL-2 therapy in a murine renal adenocarcinoma. Cell Immunol 1995; 165:243 - 51; http://dx.doi.org/10.1006/cimm.1995.1211; PMID: 7553889
- Seetharam S, Staba MJ, Schumm LP, Schreiber K, Schreiber H, Kufe DW, Weichselbaum RR. Enhanced eradication of local and distant tumors by genetically produced interleukin-12 and radiation. Int J Oncol 1999; 15:769 - 73; PMID: 10493960
- Lim JY, Gerber SA, Murphy SP, Lord EM. Type I interferons induced by radiation therapy mediate recruitment and effector function of CD8 T cells. Cancer ImmunolImmunother 2014; 63:259 - 71; PMID: 24357146
- Mason KA, Ariga H, Neal R, Valdecanas D, Hunter N, Krieg AM, Whisnant JK, Milas L. Targeting toll-like receptor 9 with CpGoligodeoxynucleotides enhances tumor response to fractionated radiotherapy. Clin Cancer Res 2005; 11:361 - 9; PMID: 15671567
- Chakravarty PK, Alfieri A, Thomas EK, Beri V, Tanaka KE, Vikram B, Guha C. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res 1999; 59:6028 - 32; PMID: 10626784
- Chen Z, Xia D, Bi X, Saxena A, Sidhu N, El-Gayed A, Xiang J. Combined radiation therapy and dendritic cell vaccine for treating solid tumors with liver micro-metastasis. J Gene Med 2005; 7:506 - 17; http://dx.doi.org/10.1002/jgm.692; PMID: 15580588
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity 2002; 17:211 - 20; http://dx.doi.org/10.1016/S1074-7613(02)00365-5; PMID: 12196292
- Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 2006; 4:423 - 34; http://dx.doi.org/10.1038/nrmicro1413; PMID: 16710323
- Brockstedt DG, Giedlin MA, Leong ML, Bahjat KS, Gao Y, Luckett W, Liu W, Cook DN, Portnoy DA, Dubensky TW Jr.. Listeria-based cancer vaccines that segregate immunogenicity from toxicity. ProcNatlAcadSci U S A 2004; 101:13832 - 7; http://dx.doi.org/10.1073/pnas.0406035101; PMID: 15365184
- Starks H, Bruhn KW, Shen H, Barry RA, Dubensky TW, Brockstedt D, Hinrichs DJ, Higgins DE, Miller JF, Giedlin M, et al. Listeria monocytogenes as a vaccine vector: virulence attenuation or existing antivector immunity does not diminish therapeutic efficacy. J Immunol 2004; 173:420 - 7; http://dx.doi.org/10.4049/jimmunol.173.1.420; PMID: 15210801
- Le DT, Brockstedt DG, Nir-Paz R, Hampl J, Mathur S, Nemunaitis J, Sterman DH, Hassan R, Lutz E, Moyer B, et al. A live-attenuated Listeria vaccine (ANZ-100) and a live-attenuated Listeria vaccine expressing mesothelin (CRS-207) for advanced cancers: phase I studies of safety and immune induction. Clin Cancer Res 2012; 18:858 - 68; http://dx.doi.org/10.1158/1078-0432.CCR-11-2121; PMID: 22147941
- DungT. Le AW-G, Vincent Picozzi, Jr, Tim F. Greten, Todd S. Crocenzi, Gregory M. Springett, Michael Morse, Herbert Zeh, Deirdre Jill Cohen, Robert Lance Fine, Beth Onners, Jennifer N. Uram, Dan Laheru, Aimee Murphy, Justin Skoble, Ed Lemmens, John J. Grous, Thomas Dubensky, Dirk G. Brockstedt, Elizabeth M. Jaffee. A phase 2, randomized trial of GVAX pancreas and CRS-207 immunotherapy versus GVAX alone in patients with metastatic pancreatic adenocarcinoma: Updated results. 2014 ASCO Gastrointestinal Cancers Symposium. San Francisco, CA, 2014.
- Kline J, Zhang L, Battaglia L, Cohen KS, Gajewski TF. Cellular and molecular requirements for rejection of B16 melanoma in the setting of regulatory T cell depletion and homeostatic proliferation. J Immunol 2012; 188:2630 - 42; http://dx.doi.org/10.4049/jimmunol.1100845; PMID: 22312128
- Hannan R, Zhang H, Wallecha A, Singh R, Liu L, Cohen P, Alfieri A, Rothman J, Guha C. Combined immunotherapy with Listeria monocytogenes-based PSA vaccine and radiation therapy leads to a therapeutic response in a murine model of prostate cancer. Cancer ImmunolImmunother 2012; 61:2227 - 38; http://dx.doi.org/10.1007/s00262-012-1257-x; PMID: 22644735
- Kärre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 1986; 319:675 - 8; http://dx.doi.org/10.1038/319675a0; PMID: 3951539
- Anfossi N, André P, Guia S, Falk CS, Roetynck S, Stewart CA, Breso V, Frassati C, Reviron D, Middleton D, et al. Human NK cell education by inhibitory receptors for MHC class I. Immunity 2006; 25:331 - 42; http://dx.doi.org/10.1016/j.immuni.2006.06.013; PMID: 16901727
- Ljunggren HG, Kärre K. In search of the ‘missing self’: MHC molecules and NK cell recognition. Immunol Today 1990; 11:237 - 44; http://dx.doi.org/10.1016/0167-5699(90)90097-S; PMID: 2201309
- Formenti SC, Demaria S. Radiation therapy to convert the tumor into an in situ vaccine. Int J RadiatOncolBiolPhys 2012; 84:879 - 80; http://dx.doi.org/10.1016/j.ijrobp.2012.06.020; PMID: 23078897
- Brown JM, Koong AC. High-dose single-fraction radiotherapy: exploiting a new biology?. Int J RadiatOncolBiolPhys 2008; 71:324 - 5; http://dx.doi.org/10.1016/j.ijrobp.2008.02.003; PMID: 18474308
- Chang JY, Liu YH, Zhu Z, Welsh JW, Gomez DR, Komaki R, Roth JA, Swisher SG. Stereotactic ablative radiotherapy: a potentially curable approach to early stage multiple primary lung cancer. Cancer 2013; 119:3402 - 10; http://dx.doi.org/10.1002/cncr.28217; PMID: 23798353
- Fidler IJ. Selection of successive tumour lines for metastasis. Nat New Biol 1973; 242:148 - 9; http://dx.doi.org/10.1038/newbio242148a0; PMID: 4512654
- Brown DM, Fisher TL, Wei C, Frelinger JG, Lord EM. Tumours can act as adjuvants for humoral immunity. Immunology 2001; 102:486 - 97; http://dx.doi.org/10.1046/j.1365-2567.2001.01213.x; PMID: 11328383
- Sorensen EW, Gerber SA, Frelinger JG, Lord EM. IL-12 suppresses vascular endothelial growth factor receptor 3 expression on tumor vessels by two distinct IFN-gamma-dependent mechanisms. J Immunol 2010; 184:1858 - 66; http://dx.doi.org/10.4049/jimmunol.0903210; PMID: 20061409
- Gerber SA, Pober JS. IFN-alpha induces transcription of hypoxia-inducible factor-1alpha to inhibit proliferation of human endothelial cells. J Immunol 2008; 181:1052 - 62; http://dx.doi.org/10.4049/jimmunol.181.2.1052; PMID: 18606657
- Gerber SA, Sorensen EW, Sedlacek AL, Lim JY, Skrombolas D, Frelinger JG, Lord EM. Local expression of interleukin-2 by B16 melanoma cells results in decreased tumour growth and long-term tumour dormancy. Immunology 2013; 138:280 - 92; http://dx.doi.org/10.1111/imm.12037; PMID: 23198850
