Abstract
The DNA damage response (DDR) upregulates the expression of NKG2D ligands (NKG2DLs).1,2 We have recently reported that the DDR also induces the presence of cytosolic DNA in B-cell lymphoma cells, which leads to the activation of STING-dependent cytosolic DNA sensor pathways and the expression of RAE-1 ligands for NKG2D.3
Natural killer group 2, member D (NKG2D, also known as KLRK1) belongs to the C-type lectin-like receptors expressed by natural killer (NK) cells, activated CD8+ T cells, CD4+ T cells under certain conditions and subsets of NKT cells and γδ+ T cells.Citation1 NKG2D contributes to the activation of NK cells and is one of the main receptors required for lysis of tumor cell lines by NK cells. In CD8+ T cells, NKG2D provides a co-stimulatory signal that enhances T-cell immune responsiveness. The ligands for NKG2D are distant relatives of class I major histocompatibility complex (MHC) molecules. In mice, many NKG2DLs belong to the family of retinoic acid early inducible-1 (RAE-1) proteins (RAE-1α-ε). In addition, three isoforms of the minor histocompatibility antigen H60 and the murine UL16-binding protein like transcript-1 (MULT-1) bind to NKG2D. NKG2D ligands are absent or expressed at low levels on normal cells. In contrast, tumor cells and virus-infected cells frequently upregulate the cell surface expression of several NKG2DLs.
DNA Damage Response
Constant surveillance of genomic insults by the DNA damage response (DDR) is critical to ensure genome integrity and suppression of tumorigenesis, as the genome is continuously exposed to a plethora of exogenous and endogenous DNA damage.Citation4 The DDR is initiated by the ataxia telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related (ATR) kinases, which govern a complex network of effector pathways including apoptosis, cell cycle arrest and DNA repair.
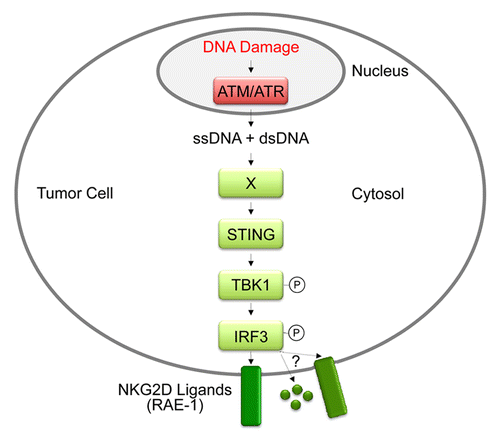
The DDR also plays an important role in the immune surveillance of cancer.Citation1 We recently reported that the DDR induces the expression of NKG2DLs through the activation of Sting-dependent cytosolic DNA sensor pathways.Citation3 Strikingly, our data suggest that DNA damage in lymphoma cells leads to the presence of single-stranded (ss) and double-stranded (ds) DNA in the cytosol (). It is conceivable that cytosolic DNA is released by dysfunctional mitochondria upon DNA damage or generated during repair of damaged genomic DNA. Inhibition of ATM and ATR, which are mostly nuclear proteins, led to the disappearance of cytosolic DNA indicating that nuclear DNA repair pathways are required for the generation of cytosolic DNA. The DDR is constitutively active in many tumor cells possibly due to oncogene-induced replication stress resulting in collapsed replication forks and associated DNA damage.Citation6 DDR-dependent homologous recombination plays an important role in restarting collapsed forks. Deletion of genomic DNA might result from homologous recombination between dispersed homologous sequences. In summary, our data suggest that nuclear DNA damage results in the presence of DNA in the cytosol of lymphoma cells.
Figure 1. STING-dependent DNA sensor pathways induce the expression of ligands for NKG2D in B-cell lymphoma cells. Damaged DNA and the ensuing DNA damage response lead to the presence of single-stranded and double-stranded DNA in the cytosol. Cytosolic DNA activates STING-dependent DNA sensor pathways, which induce the expression of NKG2D ligands and potentially other immunomodulatory molecules.
STING-Dependent Cytosolic DNA Sensor Pathway
In the presence of cytosolic dsDNA the stimulator of interferon genes (STING, officially known as TMEM173) recruits tank-binding kinase 1 (TBK1), which then phosphorylates and activates interferon regulatory factor 3 (IRF3), a transcription factor required for the expression of type I interferons (IFNs) and other anti-viral proteins.Citation7 This STING-mediated anti-viral signaling pathway is also important for expression of NKG2DLs in response to DNA damage, as genetic inhibition of Sting, Tbk1 or Irf3 impaired NKG2DL expression ().Citation3 A number of STING-dependent cytosolic DNA sensors have been described to date, including ZBP1, IFI16, DDX41 and cGAS.Citation8 We found that the upregulation of NKG2DLs in response to the genotoxic drug cytosine arabinoside (Ara-C) is partially dependent upon Zbp1 in B-cell lymphoma.Citation3 In contrast, the constitutive expression of NKG2DLs in a number of tumor cells relied on Sting, but not Zbp1, suggesting that other Sting-dependent cytosolic DNA sensors may play a role in these cancer cells. STING-activating DNA sensors have previously been associated with cancer. ZBP1 has been shown to be highly upregulated in the peritoneal lining tissue of mice bearing ascites tumors.Citation9 IFI16 controls the expression of the tumor suppressor p53 and promotes apoptosis of tumor cells. DDX41 or cGAS have not been implicated in tumorigenesis, so far. Hence, STING-dependent cytosolic DNA sensor pathways may contribute to immunosurveillance of tumors through regulation of NKG2DLs. The potential role of cytosolic DNA sensors in tumorigenesis remains to be investigated.
Irf3 Regulates NKG2DL Expression in B-cell Lymphoma of Eµ-Myc Mice
To study the functional importance of Irf3-induced NKG2DL expression, we crossed Eµ-Myc mice to Irf3-deficient mice. Eµ-Myc mice overexpress c-Myc under the control of immunoglobulin heavy chain enhancer region (Eµ), analogous to human Burkitt lymphoma. Eµ-Myc mice heterozygous for Irf3 expressed no detectable NKG2DLs at the cell surface of B-cell lymphomas.Citation3 Irf3+/−Eµ-Myc mice showed a reduced survival rate and increased tumor load. In agreement with a role of Irf3-induced NKG2DL expression in tumor surveillance, B-cell lymphomas in NKG2D-deficient Eµ-Myc mice arose significantly earlier, but no change in survival was reported.Citation1 Hence, Irf3-dependent effects, other than NKG2DL expression, are likely to play a role in promoting tumor surveillance and survival of Eµ-Myc mice. Activation of IRF3 leads to the expression of type I IFNs, which play a critical role in immunosurveillance of tumors.Citation10 In addition to enhancing the anti-tumor responses of NK cells, type I IFNs are also critical for enabling cross-presentation of tumor antigens by dendritic cells.Citation10 In summary, our data suggest that the presence of cytosolic DNA in tumor cells may activate anti-viral immune pathways leading to recognition and lysis of these self-cells. Thus, activation of the cytosolic DNA sensor pathway in tumor cells may open new approaches for cancer treatment.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed
Citation: Le Bert N, Lam AR, Ho SS, Shen YJ, Liu MM, Gasser S. STING-dependent cytosolic DNA sensor pathways regulate NKG2D ligand expression. OncoImmunology 2014; 3:e29259; 10.4161/onci.29259
References
- Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol 2013; 31:413 - 41; http://dx.doi.org/10.1146/annurev-immunol-032712-095951; PMID: 23298206
- Gasser S, Orsulic S, Brown EJ, Raulet DH. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 2005; 436:1186 - 90; http://dx.doi.org/10.1038/nature03884; PMID: 15995699
- Lam AR, Le Bert N, Ho SS, Shen YJ, Tang ML, Xiong GM, Croxford JL, Koo CX, Ishii KJ, Akira S, et al. RAE1 ligands for the NKG2D receptor are regulated by STING-dependent DNA sensor pathways in lymphoma. Cancer Res 2014; 74:2193 - 203; http://dx.doi.org/10.1158/0008-5472.CAN-13-1703; PMID: 24590060
- Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature 2009; 461:1071 - 8; http://dx.doi.org/10.1038/nature08467; PMID: 19847258
- Croxford JL, Tang ML, Pan MF, Huang CW, Kamran N, Phua CM, Chng WJ, Ng SB, Raulet DH, Gasser S. ATM-dependent spontaneous regression of early Eμ-myc-induced murine B-cell leukemia depends on natural killer and T cells. Blood 2013; 121:2512 - 21; http://dx.doi.org/10.1182/blood-2012-08-449025; PMID: 23349395
- Branzei D, Foiani M. Maintaining genome stability at the replication fork. Nat Rev Mol Cell Biol 2010; 11:208 - 19; http://dx.doi.org/10.1038/nrm2852; PMID: 20177396
- Diner EJ, Vance RE. Taking the STING out of cytosolic DNA sensing. Trends Immunol 2014; 35:1 - 2; http://dx.doi.org/10.1016/j.it.2013.10.011; PMID: 24231656
- Yanai H, Savitsky D, Tamura T, Taniguchi T. Regulation of the cytosolic DNA-sensing system in innate immunity: a current view. Curr Opin Immunol 2009; 21:17 - 22; http://dx.doi.org/10.1016/j.coi.2009.01.005; PMID: 19362700
- Shen YJ, Lam AR, Ho SS, Koo CX, Le Bert N, Gasser S. Cancer Pathogenesis and DNA sensing. In: Ishii KJ, Tang CK, eds. Biological DNA Sensor: The impact of nucleic acids on disease and vaccinology Elsevier, 2013:205-29.
- Dunn GP, Koebel CM, Schreiber RD. Interferons, immunity and cancer immunoediting. Nat Rev Immunol 2006; 6:836 - 48; http://dx.doi.org/10.1038/nri1961; PMID: 17063185
