Abstract
Upon analysis of 636 primary breast carcinoma patient samples, we have found that programmed cell death ligand 1 (PD-L1) mRNA expression is associated with tumor-infiltrating lymphocytes (TILs). Furthermore, PD-L1 expression and elevated TILs were associated with longer recurrence-free survival. Thus, our findings indicate that PD-L1 is prognostic in breast cancer and suggests a functional link between TILs and tumor PD-L1 upregulation.
Upregulation of programmed cell death ligand-1 (PDCD-L1, better known as PD-L1) is a key mechanism of tumor immune evasion. Binding of PD-L1 to its receptor PD-1 present in various immune cell types produces co-inhibitory signals leading to inactivation/exhaustion of tumor-infiltrating lymphocytes (TILs) in the tumor microenvironment.Citation1 Recent clinical trials show that blockade of the PD-1/PD-L1 axis using monoclonal antibodies can reactivate the antitumor immune response and induce lasting clinical benefit in nearly one third of (heavily pretreated) patients with advanced melanoma, lung, and renal cell carcinomas.Citation2,Citation3 Notably, tumor PD-L1 positivity scores determined by immunohistochemistry (IHC) were found to be predictive of response to the anti-PD-1 compound Nivolumab.Citation3 However, this has not been confirmed by other research groups and there is currently no standardized PD-L1 IHC assay. The literature regarding the prognostic role of tumor PD-L1 protein expression in cancer is contradictory and suggests that different IHC methods yield discordant results.Citation4 Furthermore, definition of PD-L1 positivity using IHC is subject to assay and interpretative subjectivity (e.g., assay conditions, staining pattern, threshold for signal detection or positivity, etc.).
In order to determine the value of tumor PD-L1 expression in breast cancer, we interrogated samples from 636 primary tumors from 2 independent retrospective collections. Cases were represented in tissue microarray (TMA) format and PD-L1 mRNA transcripts were measured using an antibody-independent in situ hybridization assay coupled to quantitative fluorescence.Citation5 We detected PD-L1 mRNA signal in nearly 55% of breast tumors and PD-L1 expression was significantly associated with elevated TILs and longer recurrence-free survival. Consistent with previous observations, increased TILs were recognized preferentially in hormone receptor negative tumors and were also linked with longer event-free survival. Association of PD-L1 expression with specific biological breast cancer subtypes was not evident in our study, but the proportion of HER2-positive and triple negative breast tumors was relatively low, as expected for an unselected population. Although it was initially suggested that tumor PD-L1 expression was a poor prognostic factor, recent studies using validated IHC assays support its association with increased TILs and better outcome in diverse tumor types including lung, colon carcinoma and melanoma.Citation4,Citation6,Citation7 This a priori counterintuitive association could be explained by the possible requirement of antitumor immune pressure to drive tumor PD-L1 upregulation in some tumors (). In fact, production of interferon γ (IFNγ) by activated TILs was found to precede and be required for PD-L1 induction in a melanoma model.Citation8 In addition, reactivation of dormant TILs upon PD-1/PD-L1 blockade could mediate the enhanced tumor cell killing and cytoreduction seen in responsive patients.
Figure 1. Modulation of PD-L1 levels in breast cancer. Diagram indicating the two major pathways for programmed cell death ligand 1 (PD-L1) upregulation in breast tumor cells. The blue arrows indicate the intracellular (‘intrinsic’) signaling pathway mediated by PI3K/AKT/mTOR activation. The red arrows depict the extracellular-induced (‘extrinsic’) pathway mediated by IFNγ production by TILs and subsequent IFNGRs/JAK/STAT signaling in tumor cells. Anergic cytotoxic T cells are ineffective eliminating tumor cells and they participate in the recruitment of (immune-inhibitory) CD4+Foxp3+ regulatory T cells through chemokine secretion. Ultimately, PD-L1 positive tumors show relatively fewer CD8+ and increased CD4+ TILs.
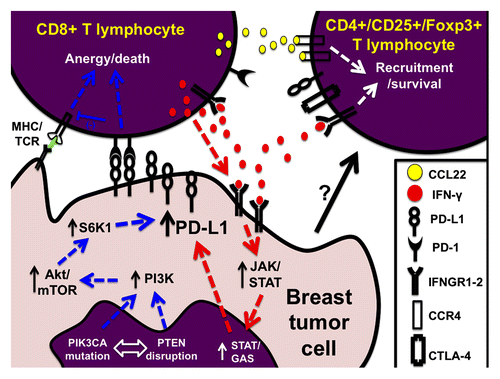
Breast cancer has not been traditionally considered as a highly immunogenic neoplasm and diverse attempts to exploit adoptive immunotherapy have, so far, shown only limited success. However, the presence of TILs and gene expression signatures representative of these cells is associated with better prognosis, particularly among high grade and hormone receptor negative tumors.Citation9 In most instances, it appears that the immune system mounts only a partially effective antitumor response insufficient to prevent disease progression. Our results indicate that only ∼16% of all breast cancers have prominent lymphocytic infiltrates and 12% show both high TILs and PD-L1 expression.Citation5 This group could represent the subset of patients with the highest potential to benefit from anti-PD-1/PD-L1 targeted therapy. In support of this notion, it was recently shown that tumors with PD-L1 expression, or with the presence of immune infiltrates, were more likely to benefit from Nivolumab treatment (48% clinical benefit in PD-L1[+] vs. 6% in PD-L1[-]; and 34% clinical benefit in the presence of immune infiltrates vs. 11% in the absence of inflammatory cells).Citation10 Our efforts to characterize the TILs subpopulations in PD-L1 expressing and non-expressing breast tumors using a multiplexed immunofluorescence assay showed increased CD3+ T and CD20+ B lymphocytes in PD-L1 expressers, but minimal change in CD8+ cytotoxic T cells.Citation5 This suggests an increase in CD4+ T cells possibly due to the preferential inhibition of cytotoxic lymphocytes as a consequence of tumor PD-L1 upregulation. Only a small proportion of breast tumors (4.7%) presented with increased TILs and absent PD-L1. This subgroup could have upregulation of alternative co-inhibitory checkpoints such as PD-L2, B7-h3, B7-h4, or LAG-3/MHCII. Importantly, the majority of breast cancers (46%) showed PD-L1 expression without prominent TILs. Altered intracellular signaling pathways, such as the stimulation of the phosphatidylinositol 3-kinase (PI3K) pathway via PIK3CA-hyperactivation and/or PTEN-reduction, could be responsible for PD-L1 expression in this group. Activation of the PI3K pathway induces PD-L1 in breast cancer cell lines and it also represents the most frequently altered signaling pathway in breast cancer (). Finally, the absence of both TILs and PD-L1 expression was seen in 37% of breast tumors. The biological determinants of this phenotype are currently unknown, but the use of multimodal strategies aiming to enhance the inflammatory infiltration (e.g., IL-2, IFNγ, vaccines, or radio-chemotherapy) together or before PD-1/PD-L1 blockade could represent promising treatment options for this scenario.
One intriguing finding of our study was the nonlinear relationship between the levels of PD-L1 protein as measured using the validated antibody clone 5h1 and the in situ PD-L1 mRNA assay.Citation5 Although the latter could be partially explained by methodological differences, the possibility of altered mRNA synthesis/translation or PD-L1 protein processing/degradation in tumor cells cannot be excluded. The observation could also be influenced by differential mRNA degradation due to pre-analytic variables. In addition, somatic variants in the PD-L1 coding gene have been detected in cancer specimens.
The in situ mRNA assay validated in our study allows measurement of relatively low-abundance transcripts in conventional formalin-fixed paraffin-embedded tissue samples through a novel hybridization strategy using multiple paired-probes and a branched signal amplification system. The key advantages of this approach as compared with RT-PCR-based methods include the preservation of tissue architecture, objective target measurement in user-defined tissue compartments (e.g., tumor cells only) and the use of a negative control for precise identification of the signal detection threshold. This method could provide a valuable tool to determine PD-L1 status in tumor tissues for clinical trials and eventually, for treatment decision.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39:1 - 10; http://dx.doi.org/10.1016/j.immuni.2013.07.012; PMID: 23890059
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012; 366:2455 - 65; http://dx.doi.org/10.1056/NEJMoa1200694; PMID: 22658128
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012; 366:2443 - 54; http://dx.doi.org/10.1056/NEJMoa1200690; PMID: 22658127
- Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest 2014; 94:107 - 16; http://dx.doi.org/10.1038/labinvest.2013.130; PMID: 24217091
- Schalper KA, Velcheti V, Carvajal D, Wimberly H, Brown J, Pusztai L, Rimm DL. In Situ Tumor PD-L1 mRNA Expression Is Associated with Increased TILs and Better Outcome in Breast Carcinomas. Clin Cancer Res 2014; 20:2773 - 82; http://dx.doi.org/10.1158/1078-0432.CCR-13-2702; PMID: 24647569
- Droeser RA, Hirt C, Viehl CT, Frey DM, Nebiker C, Huber X, Zlobec I, Eppenberger-Castori S, Tzankov A, Rosso R, et al. Clinical impact of programmed cell death ligand 1 expression in colorectal cancer. Eur J Cancer 2013; 49:2233 - 42; http://dx.doi.org/10.1016/j.ejca.2013.02.015; PMID: 23478000
- Taube JM, Anders RA, Young GD, Xu H, Sharma R, McMiller TL, Chen S, Klein AP, Pardoll DM, Topalian SL, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Transl Med 2012; 4:27ra37; http://dx.doi.org/10.1126/scitranslmed.3003689; PMID: 22461641
- Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, Gajewski TF. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med 2013; 5:ra116; http://dx.doi.org/10.1126/scitranslmed.3006504; PMID: 23986400
- Loi S, Michiels S, Salgado R, Sirtaine N, Jose V, Fumagalli D, Kellokumpu-Lehtinen PL, Bono P, Kataja V, Desmedt C, et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann Oncol 2014; Forthcoming http://dx.doi.org/10.1093/annonc/mdu112; PMID: 24608200
- Taube JM, Klein AP, Brahmer JR, Xu H, Pan X, Kim JH, Chen L, Pardoll DM, Topalian SL, Anders RA. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res 2014; Forthcoming http://dx.doi.org/10.1158/1078-0432.CCR-13-3271; PMID: 24714771
