Abstract
Neoantigen-based cancer vaccines designed to target the unique immunogenic mutations arising in each patient’s tumor are breathing new life into a struggling approach. Data continue to demonstrate the importance of neoantigens in immune control of cancer. Despite manufacturing complexity, outstanding questions and desired further improvements, neoantigen vaccines are currently undergoing clinical evaluation.
Many long-standing problems in science, medicine, or engineering are solved when new approaches become possible and are ardently applied. After many years of best efforts and countless dollars, but unmet expectations, cancer vaccines have become a long-standing problem. We and others have proposed that neoantigens – a newly available class of immunogens based on the personal, exquisitely tumor-specific mutations found uniquely in each tumor – may be the paradigm shift needed for cancer vaccines ().Citation1,Citation2
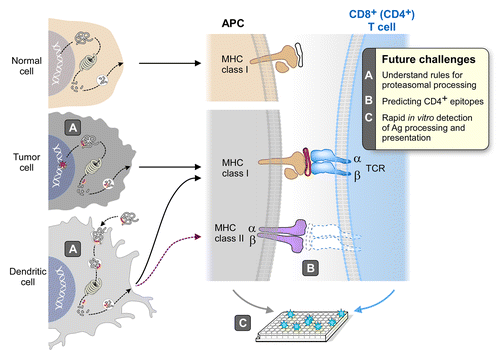
Figure 1. The neoantigen vaccine immunotherapeutic concept. Cancer cell genomes are now known to contain many mutations, some of which create amino acid changes in the encoded proteins. Some of these modified proteins will be partially degraded by the normal cellular re-cycling machinery, creating short 8 to 12 amino acid peptides, and some of these peptides will bind to one of the class I MHC molecules of the individual. Peptide binding to MHC is a critical gateway to both the initiation of a T-cell immune response by the antigen presenting cell (APC), and to the detection and elimination of tumor cells presenting the particular peptide by the stimulated cytotoxic T lymphocyte (CTL). The attraction of neoantigens as cancer targets for the immune system results from the structural and geographical features of the mutation. Central tolerance is known to purge the vast developing T-cell population in the thymus of high avidity T-cell receptors (TCRs) that recognize MHC complexes with native peptides (‘self’). In contrast, high avidity T cells are not centrally deleted against mutated peptide/MHC complexes (like viral antigens) because the mutated antigens are not present in thymus during T-cell development. These high avidity cytotoxic T cells can be selectively amplified and stimulated to attack and kill cells that present mutated peptides. Since neoantigen peptides are only found in tumor cells, the CTLs should show exquisite specificity, reducing the opportunity for autoimmune disease. Desired improvements in the process are shown in the box in the upper right.
Developing novel approaches requires commitment to a path without precedent and engaging in activities that systematically contribute to maintaining the momentum needed for change - capturing information to support the concept, raising, but not being paralyzed by, potential challenges, and identifying current shortcomings in knowledge or capabilities. In the following three sections we: (1) identify new results that continue to encourage the development of neoantigen vaccines; (2) highlight potential shortfalls of a neoantigen approach, and (3) point out areas where we challenge immunologists, biochemists and bioinformaticians to expand our basic knowledge and capabilities to help realize this novel opportunity for treatment of such a dreaded disease.
New Information
Data supporting the critical role of neoantigens in immune control has continued to build.
Neoantigens represent dominant targets in tumor-infiltrating lymphocyte (TIL) populations in patients benefiting from adoptive therapy and a neoantigen-specific population was sufficient to induce tumor regression in mouse and manCitation3-Citation6 (T. Schumacher, personal communication).
The widespread detection of spontaneously occurring neoantigen-specific T cellsCitation5,Citation7,Citation8 demonstrates that processing and presentation of multiple neoantigens does occur, despite the current insensitivity of biochemical detection on tumors.
Checkpoint blockade therapy has revealed new and amplified neoantigen-specific responses which, in the mouse, are central to disease controlCitation8 (R. Schreiber, personal communication).
Building on our previous results that T cells in autologous leukemia cell vaccinated patients recognize tumor-specific antigens,Citation9 we have identified a neoantigen as one of these targets10.
Our comprehensive literature analysis of multiple spontaneous human neoantigen responses showed that all were predictable using available algorithms for MHC binding and provided guidance for epitope selectionCitation7.
A retrospective meta-analysis of six tumor types showed that overall survival was improved in patients predicted to have at least one immunogenic neoantigen epitope.Citation11
Two clinical trials have now been initiated to directly test the concept of a neoantigen vaccine (NCT01970358 and NCT02035956).
Valid Questions
Guilty until proven innocent is a valid scientific philosophy and so multiple questions challenge the viability of a neoantigen vaccine.
By the time cancer is detected, would a vaccine approach have lost its chance due to local and systemic immune suppression? Paradoxically, the success of checkpoint blockade in producing durable remission in a significant subset of patients has both validated the physiological importance of immune suppression but also validated that pharmacological intervention can shift that delicate balance toward tumor control. Correspondingly, we posit that the spontaneous immune reaction to a slowly growing and evolving tumor is likely sub-optimal, especially in the absence of appropriate ‘danger’ signals, and that a multi-epitope, neoantigen-based vaccine delivered with a powerful adjuvant has the ability to productively impact the clinical response.
Is a multi-epitope, personalized vaccine too impractical to manufacture for widespread use? We reject this barrier to innovation. Interwoven key concerns frequently raised include cost, timing, and good manufacturing practice (GMP). Ultimately, engineering, focused technology development and logistics will solve these problems if clinical benefit is demonstrated. The cost of sequencing continues to fall and a properly scaled and designed process along with a streamlined approach to product assays can maintain manufacturing time and cost within acceptable limits. Finally, regulatory authorities have always responded to exciting new therapeutic opportunities with cooperation and adaptability.
Will genetic heterogeneity within the tumor always result in cells resistant to immune attack? Tumor evolution is driven by multiple independent pressures, including immune pressure, and results in genetic variation over time and space. Heterogeneity is thus a primary rationale for utilizing multiple independent epitopes in a vaccine, reducing the possibility of immune escape due to downregulation of a single antigen.
A Call to Arms
A silver lining, if there can be one, of the failure of past cancer vaccine approaches has been the continual improvement to vaccine strategies. We further encourage work in the following areas to strengthen our understanding of antigen processing, presentation and detection and thus maximize the potential of this novel immunogen class:
Discovering the rules for proteasomal processing in professional APCs and tumor cells to provide in silico filters to complement MHC binding predictions.
Developing the capability to predict which mutated peptides are presented by MHC class II to CD4+ T cells. CD4+ T cell help against tumor-expressed neoantigens may be important for stimulating local responses within the tumor.
Developing rapid assays to measure candidate neoantigen processing and presentation by APC and the subsequent activation of T cells ex vivo. Such assays will help to refine rules of processing and may provide real-time guidance in epitope selection.
Conclusion
The recent failure of the MAGE-A3 vaccine to meet the first two primary endpoints in its non-small cell lung cancer trial sent chills through an already stymied set of cancer vaccine proponents. The old adage ‘If wishes were horses, then beggars would ride’ is a sobering reminder that to move forward we need results. We anticipate that neoantigen vaccination will provide such results by fostering anticancer immunity.
Disclosures of Potential Conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Ute E. Burkhardt for helpful suggestions to the manuscript.
References
- Heemskerk B, Kvistborg P, Schumacher TN. The cancer antigenome. EMBO J 2013; 32:194 - 203; http://dx.doi.org/10.1038/emboj.2012.333; PMID: 23258224
- Hacohen N, Fritsch EF, Carter TA, Lander ES, Wu CJ. Getting personal with neoantigen-based therapeutic cancer vaccines. [Cancer Immunology at the Crossroads] Cancer Immunol Res 2013; 1:11 - 5; http://dx.doi.org/10.1158/2326-6066.CIR-13-0022; PMID: 24777245
- Robbins PF, Lu YC, El-Gamil M, Li YF, Gross C, Gartner J, Lin JC, Teer JK, Cliften P, Tycksen E, et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med 2013; 19:747 - 52; http://dx.doi.org/10.1038/nm.3161; PMID: 23644516
- Wick DA, Webb JR, Nielsen JS, Martin SD, Kroeger DR, Milne K, Castellarin M, Twumasi-Boateng K, Watson PH, Holt RA, et al. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin Cancer Res 2014; 20:1125 - 34; http://dx.doi.org/10.1158/1078-0432.CCR-13-2147; PMID: 24323902
- Gros A, Robbins PF, Yao X, Li YF, Turcotte S, Tran E, Wunderlich JR, Mixon A, Farid S, Dudley ME, et al. PD-1 identifies the patient-specific CD8⁺ tumor-reactive repertoire infiltrating human tumors. J Clin Invest 2014; 124:2246 - 59; http://dx.doi.org/10.1172/JCI73639; PMID: 24667641
- Tran E, Turcotte S, Gros A, Robbins PF, Lu Y-C, Dudley ME, Wunderlich JR, Somerville RP, Hogan K, Hinrichs CS, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 2014; 344:641 - 5; http://dx.doi.org/10.1126/science.1251102; PMID: 24812403
- Fritsch EF, Rajasagi M, Ott PA, Brusic V, Hacohen N, Wu CJ. HLA-binding properties of tumor neoepitopes in humans. Cancer Immunology Research 2014.
- van Rooij N, van Buuren MM, Philips D, Velds A, Toebes M, Heemskerk B, van Dijk LJ, Behjati S, Hilkmann H, El Atmioui D, et al. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol 2013; 31:e439 - 42; http://dx.doi.org/10.1200/JCO.2012.47.7521; PMID: 24043743
- Burkhardt UE, Hainz U, Stevenson K, Goldstein NR, Pasek M, Naito M, Wu D, Ho VT, Alonso A, Hammond NN, et al. Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells. J Clin Invest 2013; 123:3756 - 65; http://dx.doi.org/10.1172/JCI69098; PMID: 23912587
- Rajasagi M, Shukla SA, Fritsch EF, Keskin DB, DeLuca D, Carmona E, Zhang W, Sougnez C, Cibulskis K, Sidney J, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood 2014; 124:453 - 62; http://dx.doi.org/10.1182/blood-2014-04-567933; PMID: 24891321
- Brown SD, Warren RL, Gibb EA, Martin SD, Spinelli JJ, Nelson BH, Holt RA. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res 2014; 24:743 - 50; http://dx.doi.org/10.1101/gr.165985.113; PMID: 24782321
