Abstract
We recently reported that tumor-directed antibodies could either stimulate, or inhibit, tumor progression, dependent upon the dosage used. The narrow range over which this immune response curve (IRC) occurs is surprising. Here we discuss features of the IRC, the mechanisms identified so far, and the potential clinical implications.
A large body of literature demonstrates that immune reactants can be used to inhibit and kill cancers.Citation1 This forms the robust foundation for cancer immunotherapy. On the contrary, there are also many examples whereby immune reactants, including immune cells and antibodies within the tumor microenvironment are tumor stimulating.Citation2-Citation4 This has commonly led to the immune response to cancer being described as a ‘double-edged sword,’ where immunosurveillance of the tumor is on one edge and the tumor promoting inflammation is on the other edge. In our recent reportCitation5 we found that a particular class of tumor-directed immune reactants, anticancer antibodies, stimulated tumor growth at low doses and inhibited growth at higher doses. Thus, there is not only a dichotomy of one or the other edge, but it also matters how hard the ‘inflammatory sword hits,’ in determining whether tumor growth is stimulated or inhibited. This allowed us to define an immune response curve (IRC, ), which was first suggested by Richmond Prehn. In a 2010 update,Citation6 Prehn predicted that while a low quantity of immune reactant(s) against a growing tumor might be stimulatory, higher quantities of the same immune reactant(s) would inhibit tumor growth. Our work experimentally demonstrates a role for antitumor antibodies that fits this hypothesis.
Figure 1. The immune response curve to antibody-based anticancer therapeutics. Very low levels of tumor-directed antibody (Zone A) have no effect on tumor growth, but as this dose increases (red zone B-D) tumor growth is stimulated via activation of PI3K/AKT pathway, and high infiltration of M2 polarized macrophages. There is a dose of antibody that generates a maximum stimulatory effect (Zone C). Increasing the dose of antibody finally leads to tumor growth inhibition (Zone E to F) via natural killer (NK) cell mediated antibody-dependent complement cascade (ADCC) and complement-mediated tumor cell lysis. However, this curve goes through a null zone, suggesting there is a dose of antibody that generates stimulatory and inhibitory mechanisms in quantities that mutually cancel each other out to give no net overall effect on tumor growth.
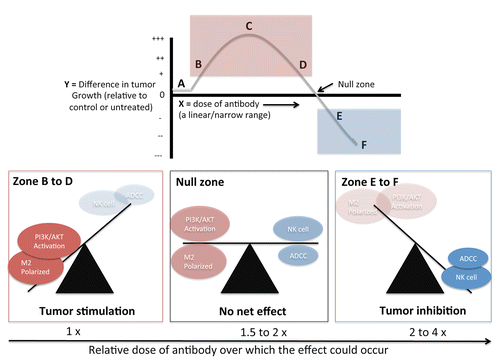
The IRC we have generated using multiple murine models yielded a surprisingly narrow and linear range of antibody doses spanning this binary response.Citation5 This work also allowed us to investigate another unanswered question regarding the mechanism of tumor inhibition or promotion by the immune system. While it is well established that cancer-associated immune reactions can be either stimulatory or inhibitory, it is not so clear whether the mechanisms that govern this effect are separate, or an overlapping balance of multiple variables.Citation7 We found that low, stimulatory doses of antibody corresponded with a significant increase in macrophage infiltration, consisting of tumor-promoting M2-polarized macrophages. Depletion of macrophages blocked the stimulatory effects of the low dose antibody. On the other hand, high, inhibitory doses of antibody showed a marked reduction in macrophage infiltration and a decrease of M2 polarization. (For a review of tumor-associated immune cell polarization see ref. Citation8). Under inhibitory doses we saw an increased natural killer (NK) cell infiltration, and depletion of NK cells blocked the inhibitory effects.
The data suggests that in our model the cellular mechanism by which a low dose stimulated and a high dose inhibited were separate. However, we found that increasing the dose of antibody above stimulatory levels passed through a ‘null’ zone (), where there is no net effect on tumor growth. Increasing the dose of antibody from this zone leads to inhibited tumor growth. This suggests therefore that there is a point at which these disparate mechanisms of stimulation and inhibition overlap and cancel each other out leading to no net effect. We also noted that this effect of stimulation or inhibition could occur independently of any adaptive immunity. While this work was under review a separate study was published showing similar immune response curves could be drawn using a selection of complement-activating antibodies.Citation4 In this example, inhibition was via direct lysis of tumor cells via complement activation, and stimulation with low sub-lytic antibody dose was shown to be dependent on activation of the PI3K/AKT survival pathway. Together these two highly complementary studies make a case for immunoglobulins as tumor stimulators and inhibitors in a dose dependent manner, both suggesting that the underlying mechanism of stimulation or inhibition are separate, but overlapping. Not discussed here is how both studies fit into the larger field of hormesis and medicine (for an overview we refer to Calabrese et al.Citation9).
The clinical implications of the IRC and immunotherapies of cancer are not yet clear, but there are potential considerations that could benefit cancer patients. For instance, up to 10% of patients undergoing rituximab monotherapy for low-grade CD20 positive B-cell lymphoma will show progressive disease shortly after the first antibody administration,Citation10 which could potentially be due to areas of low antibody concentrations within a tumor that support tumor growth rather than suppressing it, although other potential mechanisms of primary therapy resistance have also been described. It’s worth noting that immunotherapies that potentially stimulate tumor growth may at the same time sensitize a tumor to chemotherapy, and therefore the net effect would still be beneficial. The IRC could have its biggest impact in maintenance regimes, since the IRC would predict that as the dose of reactant falls the chances for a tumor stimulatory effect would increase.
Our work suggests that the immune reactions toward cancer cannot only be described as a double-edged sword but in the case of antibodies rather as a one-edged Samurai sword that stimulates or inhibits tumor growth depending how hard it strikes (quantity and quality of antibody). While the mechanisms of tumor inhibition and promotion might be distinct, further studies are needed to better understand this phenomenon.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature 2011; 480:480 - 9; http://dx.doi.org/10.1038/nature10673; PMID: 22193102
- Balkwill FR, Capasso M, Hagemann T. The tumor microenvironment at a glance. J Cell Sci 2012; 125:5591 - 6; http://dx.doi.org/10.1242/jcs.116392; PMID: 23420197
- Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell 2010; 17:121 - 34; http://dx.doi.org/10.1016/j.ccr.2009.12.019; PMID: 20138013
- Wu X, Ragupathi G, Panageas K, Hong F, Livingston PO. Accelerated tumor growth mediated by sublytic levels of antibody-induced complement activation is associated with activation of the PI3K/AKT survival pathway. Clin Cancer Res 2013; 19:4728 - 39; http://dx.doi.org/10.1158/1078-0432.CCR-13-0088; PMID: 23833306
- Pearce OM, Läubli H, Verhagen A, Secrest P, Zhang J, Varki NM, Crocker PR, Bui JD, Varki A. Inverse hormesis of cancer growth mediated by narrow ranges of tumor-directed antibodies. Proc Natl Acad Sci U S A 2014; 111:5998 - 6003; http://dx.doi.org/10.1073/pnas.1209067111; PMID: 24711415
- Prehn RT. The initial immune reaction to a new tumor antigen is always stimulatory and probably necessary for the tumor’s growth. Clin Dev Immunol 2010; 2010; http://dx.doi.org/10.1155/2010/851728; PMID: 20811480
- Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: independent or interdependent processes?. Curr Opin Immunol 2007; 19:203 - 8; http://dx.doi.org/10.1016/j.coi.2007.02.001; PMID: 17292599
- Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol 2008; 18:349 - 55; http://dx.doi.org/10.1016/j.semcancer.2008.03.004; PMID: 18467122
- Calabrese EJ. Hormesis and medicine. Br J Clin Pharmacol 2008; 66:594 - 617; PMID: 18662293
- Hainsworth JD, Burris HA 3rd, Morrissey LH, Litchy S, Scullin DC Jr., Bearden JD 3rd, Richards P, Greco FA. Rituximab monoclonal antibody as initial systemic therapy for patients with low-grade non-Hodgkin lymphoma. Blood 2000; 95:3052 - 6; PMID: 10807768
