Abstract
After protein phosphorylation on certain serine or threonine residues preceding a proline (pSer/Thr-Pro), the function of certain phosphorylated protein is further regulated by cis-trans conformational change. Due to the lack of any tool to detect such two conformations in cells, however, it is not even known whether any cis or trans conformation exists in vivo, not to mention their conformation-specific functions or regulation. We developed a novel peptide chemistry technology to generate the first pair of antibodies that can distinguish cis from trans pThr231-Pro tau. Cis, but not trans, pThr231-tau appears early in mild cognitive impairment (MCI) neurons and further accumulates in only degenerating neurons as Alzheimer disease (AD) progresses, localizing to dystrophic neurites, which are known to correlate well with memory loss. Unlike trans p-tau, the cis cannot promote microtubule assembly, and is more resistant to dephosphorylation and degradation and more prone to aggregation. Pin1 accelerates cis to trans isomerization to prevent tau pathology in AD. Thus, during MCI and AD development, cis pThr231-Pro tau is the earliest detectable pathogenic tau conformation and antibodies and vaccines against the pathogenic cis p-tau may be used for the early diagnosis and treatment of AD. These findings offer in vivo approach to study conformational regulation of Pro-directed phosphorylation signaling.
The reversible protein phosphorylation on certain serine or threonine residues preceding a proline (pSer/Thr-Pro) is a crucial signaling mechanism in various cellular processes in physiological and pathological conditions.Citation1 Certain proline residues in proteins can exist in completely distinct cis and trans peptide bond conformations and provide an intrinsic backbone switch.Citation2 Conversion between cis and trans is markedly slowed down upon phosphorylation, but specifically accelerated by the unique prolyl isomerase Pin1, which has a profound impact on a spectrum of activities in many signaling molecules.Citation2,Citation3
Specific functions of proline-directed cis and trans conformers have been proposed by various indirect approaches. The first strategy to dissect distinct functions of the proline isomers is molecular simulation, that is, determination of structures that enable specific functions such as distances between two critical amino acids.Citation4-Citation6 The second approach is to synthesize mutant proteins by replacing some amino acids with different amino acids that mimic cis or trans conformation.Citation7 The third procedure to determine isomer-specific functions is to substitute proline with non-natural proline analogs that have different tendency of cis and trans conformations.Citation8
However, the functions of endogenous cis and trans conformations in native proteins in vivo remain to be determined because there is not any structure available that we are aware of showing both the cis and trans conformations at one pSer/Thr-Pro motif in an intact protein, which is likely due to the difficulty in obtaining a sufficient amount of stoichiometrically phosphorylated proteins for structural analyses of such two flexible conformations. To detect endogenous cis and trans forms in proteins in vivo, we reasoned that antibodies would only recognize either cis or trans, but not both of a pSer/Thr-Pro motif in a native protein.
We generated antibodies that specifically recognize either cis or trans conformation in tau protein,Citation9 a substrate of Pin1. Pin1 acts on the pThr231-Pro motif in tauCitation10 (1) to restore the ability of p-tau to promote microtubule assembly,Citation10 (2) to facilitate p-tau dephosphorylation because PP2A is trans-specificCitation11,Citation12 and (3) to promote p-tau degradation.Citation13 Notably, T231 phosphorylation in tau is reported to be very early, if not the earliest of sequential tau phosphoepitopes appearing during early stages of AD pretangle formation.Citation14-Citation16 A key step to develop such conformation- and phospho-specific antibodies is to increase the cis content of pSer/Thr-Pro motifs in the antigen with the smallest change possible because ~90% of pSer/Thr-Pro motifs in a synthetic peptide are in trans.Citation17-Citation19 Indeed, NMR analysis showed that the pThr231-Pro motif in a synthetic tau peptide contained only 9% in the cis conformation.Citation9 Our strategy is to immunize rabbits with a modified pThr231-Pro tau peptide that contains a minimal structural change, but has both cis and trans contents high enough to produce cis- and trans-specific antibodies, followed by separating them using affinity purification and counter-purification procedures with cis and trans locked peptides, respectively. We have discovered that replacing the five-membered carbonyl ring of Pro with a six-membered ring having one additional methylene group, as seen in homoproline (Pip), increases the cis content and also produce antibodies that recognize endogenous tau proteins. Thus, we used the pThr231-Pip tau peptide that has minimal structural change as the antigen. The reason why we did not use a cis locked pThr231-Dmp (5,5-dimethylproline) and a pure trans pThr231-Ala tau peptides as antigens is that it remains to be determined whether the resulting antibodies could recognize endogenous tau because these peptides have much bigger structural changes than the pThr231-Pip peptide. Instead, we used the cis locked and pure trans peptides for subsequent separation of cis- and trans-specific antibodies that have already produced.
Using these antibodies, we have provided multiple lines of evidence showing distinct localizations and functions of the cis and trans conformations during the development of AD ().Citation9 The cis, but not trans, pThr231-tau appears very early in MCI neurons and further accumulates in degenerated neurons as AD progresses, and the cis, but not trans, localizes to the dystrophic neurites, an early hallmark that is highly correlated with cognitive loss in AD patients.Citation20-Citation22 Functionally, trans, but not cis, pThr231-tau promotes microtubule assembly. Biochemically, cis p-tau is more resistant to protein dephosphorylation and degradation, and also more prone to protein aggregation than the trans, which might explain why the cis is much more abundant in MCI and AD brains. These results are also consistent with the previous findings that the major tau phosphatase PP2A does not dephosphorylate pThr231-tau peptides where trans conformer is chemically deleted,Citation12 and that F-box proteins, which target phosphoproteins to proteasomes, bind to certain pSer/Thr-Pro motifs only in trans.Citation23 These results indicate that cis, but not trans, p-tau displays both toxic gains-of-function and losses of normal tau function, two major properties that are known to contribute to tau pathology in AD.
Figure 1. Generation of conformation-specific antibodies identifies the cis pT231-tau as the earliest detectable pathogenic conformation leading to tau pathology and memory loss in MCI and AD, and suggests cis-specific biomarker and immunotherapy for early diagnosis and treatment.
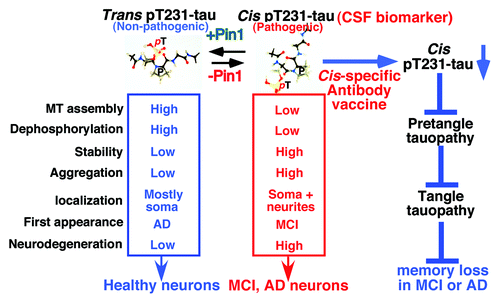
The conformation-specific antibodies also enabled to show that Pin1 regulates conversion from the cis to transin vivo.Citation9 In the AD hippocampus, the cis is inversely correlated with Pin1 levels and completely overlapped with neurofibrillary neurodegeneration, whereas the trans is positively correlated with Pin1 levels and is rarely associated with neurofibrillary neurodegeneration. Moreover, Pin1 overexpression in AD mouse models decreases the cis pThr231-tau, whereas Pin1 knockout increases the cis. These results are highly relevant to human AD because Pin1 is inhibited in MCI and AD neurons by multiple mechanismsCitation24,Citation25 and preventing Pin1 inhibition is associated with delayed onset of AD.Citation26 Given that pT231-tau in brain tissues is the earliest detectable event in the sequential appearance of tau phosphoepitopes in pretangle neurons,Citation14-Citation16 these results indicate that during the development of MCI and AD, cis, rather than trans, pThr231-tau is the earliest detectable pathogenic conformation leading to tau pathology and memory loss, but is accumulated due to lack of sufficient Pin1 to convert it to the non-toxic trans.
This exciting new insight into the role and regulation of tau protein conformations in tauopathy might have some novel therapeutic implications. Since Pin1 overexpression accelerates cis-trans isomerization, overexpressing Pin1 or preventing Pin1 inhibition might be a new approach to reduce the cis to block tauopathy at the early stage. Moreover, it has been shown that tauopathy can spread from neuron to neuronCitation27 and that activeCitation28 or passiveCitation29 immunotherapy against tangle-containing p-tau epitopes reduces tau aggregates and improves memory deficits in mouse models. Since recent studies show that neuronal dysfunction precedes tangle formation,Citation9,Citation30 a major challenge is how to develop effective p-tau immunotherapy targeting only the earliest possible pathogenic pretangle event(s) in AD. Our findings that cis, but not trans, pT231-tau is the earliest detectable pathogenic conformation that leads to tauopathy in AD suggest that conformation-specific antibodies and vaccines against only the pathogenic cis pT231-tau would be highly effective, specific and safe in stopping tauopathy and memory loss in AD and that assaying CSF cis pT231-tau would help identify appropriate patients for such cis-targeted immunotherapy and to monitor the therapeutic response.
| Abbreviations: | ||
| Alzheimer’s disease | = | AD |
| mild cognitive impairment | = | MCI |
Acknowledgments
Work done in the authors’ laboratory is supported by NIH grants R01AG039405, R01AG17870 and R01CA167677 to K.P.L. and R01CA122434 to X.Z.Z.
References
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature 2001; 411:355 - 65; http://dx.doi.org/10.1038/35077225; PMID: 11357143
- Lu KP, Finn G, Lee TH, Nicholson LK. Prolyl cis-trans isomerization as a molecular timer. Nat Chem Biol 2007; 3:619 - 29; http://dx.doi.org/10.1038/nchembio.2007.35; PMID: 17876319
- Lee TH, Pastorino L, Lu KP. Peptidyl-prolyl cis-trans isomerase Pin1 in ageing, cancer and Alzheimer disease. Expert Rev Mol Med 2011; 13:e21; http://dx.doi.org/10.1017/S1462399411001906; PMID: 21682951
- Eckert B, Martin A, Balbach J, Schmid FX. Prolyl isomerization as a molecular timer in phage infection. Nat Struct Mol Biol 2005; 12:619 - 23; http://dx.doi.org/10.1038/nsmb946; PMID: 15937494
- Sarkar P, Reichman C, Saleh T, Birge RB, Kalodimos CG. Proline cis-trans isomerization controls autoinhibition of a signaling protein. Mol Cell 2007; 25:413 - 26; http://dx.doi.org/10.1016/j.molcel.2007.01.004; PMID: 17289588
- Sarkar P, Saleh T, Tzeng SR, Birge RB, Kalodimos CG. Structural basis for regulation of the Crk signaling protein by a proline switch. Nat Chem Biol 2011; 7:51 - 7; http://dx.doi.org/10.1038/nchembio.494; PMID: 21131971
- Wang L, Yang F, Zhang D, Chen Z, Xu RM, Nierhaus KH, et al. A conserved proline switch on the ribosome facilitates the recruitment and binding of trGTPases. Nat Struct Mol Biol 2012; 19:403 - 10; http://dx.doi.org/10.1038/nsmb.2254; PMID: 22407015
- Lummis SC, Beene DL, Lee LW, Lester HA, Broadhurst RW, Dougherty DA. Cis-trans isomerization at a proline opens the pore of a neurotransmitter-gated ion channel. Nature 2005; 438:248 - 52; http://dx.doi.org/10.1038/nature04130; PMID: 16281040
- Nakamura K, Greenwood A, Binder L, Bigio EH, Denial S, Nicholson L, et al. Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer’s disease. Cell 2012; 149:232 - 44; http://dx.doi.org/10.1016/j.cell.2012.02.016; PMID: 22464332
- Lu PJ, Wulf G, Zhou XZ, Davies P, Lu KP. The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 1999; 399:784 - 8; http://dx.doi.org/10.1038/21650; PMID: 10391244
- Liou YC, Sun A, Ryo A, Zhou XZ, Yu ZX, Huang HK, et al. Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 2003; 424:556 - 61; http://dx.doi.org/10.1038/nature01832; PMID: 12891359
- Zhou XZ, Kops O, Werner A, Lu PJ, Shen M, Stoller G, et al. Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol Cell 2000; 6:873 - 83; http://dx.doi.org/10.1016/S1097-2765(05)00083-3; PMID: 11090625
- Lim J, Balastik M, Lee TH, Nakamura K, Liou YC, Sun A, et al. Pin1 has opposite effects on wild-type and P301L tau stability and tauopathy. J Clin Invest 2008; 118:1877 - 89; PMID: 18431510
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT. Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 2002; 103:26 - 35; http://dx.doi.org/10.1007/s004010100423; PMID: 11837744
- Luna-Muñoz J, Chávez-Macías L, García-Sierra F, Mena R. Earliest stages of tau conformational changes are related to the appearance of a sequence of specific phospho-dependent tau epitopes in Alzheimer’s disease. J Alzheimers Dis 2007; 12:365 - 75; PMID: 18198423
- Luna-Muñoz J, García-Sierra F, Falcón V, Menéndez I, Chávez-Macías L, Mena R. Regional conformational change involving phosphorylation of tau protein at the Thr231, precedes the structural change detected by Alz-50 antibody in Alzheimer’s disease. J Alzheimers Dis 2005; 8:29 - 41; PMID: 16155347
- Pal D, Chakrabarti P. Cis peptide bonds in proteins: residues involved, their conformations, interactions and locations. J Mol Biol 1999; 294:271 - 88; http://dx.doi.org/10.1006/jmbi.1999.3217; PMID: 10556045
- Schutkowski M, Bernhardt A, Zhou XZ, Shen M, Reimer U, Rahfeld JU, et al. Role of phosphorylation in determining the backbone dynamics of the serine/threonine-proline motif and Pin1 substrate recognition. Biochemistry 1998; 37:5566 - 75; http://dx.doi.org/10.1021/bi973060z; PMID: 9548941
- Stewart DE, Sarkar A, Wampler JE. Occurrence and role of cis peptide bonds in protein structures. J Mol Biol 1990; 214:253 - 60; http://dx.doi.org/10.1016/0022-2836(90)90159-J; PMID: 2370664
- Davies CA, Mann DM, Sumpter PQ, Yates PO. A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer’s disease. J Neurol Sci 1987; 78:151 - 64; http://dx.doi.org/10.1016/0022-510X(87)90057-8; PMID: 3572454
- DeKosky ST, Scheff SW. Synapse loss in frontal cortex biopsies in Alzheimer’s disease: correlation with cognitive severity. Ann Neurol 1990; 27:457 - 64; http://dx.doi.org/10.1002/ana.410270502; PMID: 2360787
- Scheff SW, DeKosky ST, Price DA. Quantitative assessment of cortical synaptic density in Alzheimer’s disease. Neurobiol Aging 1990; 11:29 - 37; http://dx.doi.org/10.1016/0197-4580(90)90059-9; PMID: 2325814
- Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell 2003; 112:243 - 56; http://dx.doi.org/10.1016/S0092-8674(03)00034-5; PMID: 12553912
- Segat L, Pontillo A, Annoni G, Trabattoni D, Vergani C, Clerici M, et al. PIN1 promoter polymorphisms are associated with Alzheimer’s disease. Neurobiol Aging 2007; 28:69 - 74; http://dx.doi.org/10.1016/j.neurobiolaging.2005.11.009; PMID: 16384626
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, et al. Oxidative modification and down-regulation of Pin1 in Alzheimer’s disease hippocampus: A redox proteomics analysis. Neurobiol Aging 2006; 27:918 - 25; http://dx.doi.org/10.1016/j.neurobiolaging.2005.05.005; PMID: 15950321
- Ma SL, Tang NL, Tam CW, Lui VW, Lam LC, Chiu HF, et al. A PIN1 polymorphism that prevents its suppression by AP4 associates with delayed onset of Alzheimer’s disease. Neurobiol Aging 2012; 33:804 - 13; http://dx.doi.org/10.1016/j.neurobiolaging.2010.05.018; PMID: 20580132
- Clavaguera F, Bolmont T, Crowther RA, Abramowski D, Frank S, Probst A, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nat Cell Biol 2009; 11:909 - 13; http://dx.doi.org/10.1038/ncb1901; PMID: 19503072
- Asuni AA, Boutajangout A, Quartermain D, Sigurdsson EM. Immunotherapy targeting pathological tau conformers in a tangle mouse model reduces brain pathology with associated functional improvements. J Neurosci 2007; 27:9115 - 29; http://dx.doi.org/10.1523/JNEUROSCI.2361-07.2007; PMID: 17715348
- Chai X, Wu S, Murray TK, Kinley R, Cella CV, Sims H, et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J Biol Chem 2011; 286:34457 - 67; http://dx.doi.org/10.1074/jbc.M111.229633; PMID: 21841002
- Spires-Jones TL, Kopeikina KJ, Koffie RM, de Calignon A, Hyman BT. Are tangles as toxic as they look?. J Mol Neurosci 2011; 45:438 - 44; http://dx.doi.org/10.1007/s12031-011-9566-7; PMID: 21638071