Abstract
In this study we investigated the influence of the global response regulator PhoP on the complex regulatory cascade controlling expression of early stage virulence genes of Yersinia pseudotuberculosis via the virulence regulator RovA. Our analysis revealed the following novel features: (1) PhoP activates expression of the CsrC RNA in Y. pseudotuberculosis, leading to activation of RovA synthesis through the CsrABC-RovM cascade, (2) activation of csrC transcription is direct and PhoP is shown to bind to two separate PhoP box-like sites, (3) PhoP-mediated activation results in transcription from two different promoters closely downstream of the PhoP binding sites, leading to two distinct CsrC RNAs, and (4) the stability of the CsrC RNAs differs significantly between the Y. pseudotuberculosis strains YPIII and IP32953 due to a 20 nucleotides insertion in CsrCIP32953, which renders the transcript more susceptible to degradation. In summary, our study showed that PhoP-mediated influence on the regulatory cascade controlling the Csr system and RovA in Y. pseudotuberculosis varies within the species, suggesting that the Csr system is a focal point to readjust and adapt the genus to different hosts and reservoirs.
Introduction
Pathogenic bacteria, which circulate frequently between external habitats/reservoirs and warm-blooded hosts, are continuously confronted with rapidly changing environmental conditions, including variations of nutrients and ion concentrations, temperature, oxygen, and pH. Within their hosts, the bacteria have to cope with competing microbiota and often very harmful situations, such as the attack of the host immune system aiming to eradicate invading pathogens. In order to ensure their survival and propagation, bacteria need to survey the changes of their local environment, transduce, and translate the signals to respond adequately.
In bacteria, two-component regulatory systems serve as an external stimulus-response coupling machinery to convert specific environmental signals into a cellular response, typically by differential expression of multiple target genes. Generally, a histidine protein kinase and a response regulator form the basis of these modular and adaptable phosphotransfer-signaling schemes.Citation1-Citation3 The pleiotropic PhoP/PhoQ two-component system constitutes one of the most crucial signal transduction systems controlling bacterial virulence.Citation4-Citation6 The membrane-bound sensor kinase PhoQ responds predominantly to low environmental Mg2+ (but also to Ca2+ and Mn2+), acidic pH, and host-secreted antimicrobial peptides, and phosphorylates the cytoplasmic response regulator PhoP.Citation4 Phosphorylated PhoP either activates or represses its target genes through binding to a conserved binding motif in the proximity of the target promoters,Citation7-Citation9 which is similar to the PhoP-box sequence of E. coli and Salmonella.Citation10-Citation12 Most of the work on PhoP/PhoQ and its role for virulence has been performed with Salmonella and Yersinia species. In both species, PhoP/PhoQ promotes survival and proliferation in macrophages and neutrophils, as well as survival in the presence of antimicrobial peptides.Citation4,Citation13-Citation18 A phoP knockout mutant of S. enterica serovar Typhimurium was shown to be highly attenuated for virulence in mice.Citation4,Citation14,Citation19,Citation20 The role of the PhoP/PhoQ system for virulence in Yersinia is less clear as conflicting results have been reported for different strains and infection models, but the overall defect of the phoP mutants in virulence is more modest compared with Salmonella.Citation15,Citation21-Citation24 The phenotypic differences of the phoP mutants might reflect variations in the regulatory circuits and composition of the individual regulons. In fact, the PhoP/PhoQ regulons of Y. pestis and S. enterica are both complex and include many target genes, albeit the architecture of the PhoP-dependent promoters as well as the function of PhoP itself vary considerably between the two pathogens.Citation25,Citation26
The PhoP regulon members have been identified in Y. pestis biovar Microtus, which is supposed to be avirulent to humans. Several genes, which are involved in detoxification, protection against DNA damage, resistance to antimicrobial peptides, and adaptation to magnesium limitation (e.g., mgtCB that encodes a Mg2+ transport system) were proven to be direct targets of PhoP.Citation9,Citation27,Citation28 Recent studies further showed that PhoP of Y. pestis biovar Microtus also acts as direct activator of crp and as repressor of the rovA, psaEF, and psaABC loci,Citation28,Citation29 which are all part of a complex regulatory cascade characterized in the close relative Y. pseudotuberculosis ().Citation30
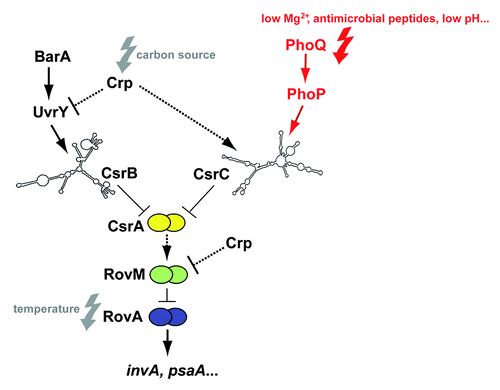
Figure 1. Regulatory cascade controlling expression of Y. pseudotuberculosis early-stage virulence genes. Expression of the virulence genes invasin (invA) and psaA is activated by the thermosensing virulence regulator RovA. Synthesis of RovA is controlled in a nutrient-dependent manner by a regulatory cascade, including the cAMP receptor protein (Crp) and the two-component regulatory proteins UvrY/BarA. They regulate components of the carbon storage regulator (Csr) system, i.e., the regulatory RNAs CsrB and CsrC and the RNA-binding protein CsrA. Upregulation of CsrB and/or CsrC leads to the sequestration of CsrA, whereby synthesis of the LysR-type repressor RovM is inhibited, which allows production of RovA. Furthermore, RovM synthesis is repressed by Crp downstream of CsrA via an unknown mechanism. The new finding that csrC expression is activated by the PhoP/PhoQ two component system is highlighted in red. Solid lines illustrate direct control, and dashed lines indirect regulation by the indicated regulator.
RovA belongs to the SlyA/Hor/Rap family of MarR-type dimeric winged-helix DNA-binding proteins, controlling a wide range of physiological processes involved in environmental and host-associated stress adaptation and virulence in bacterial pathogens.Citation31 The RovA regulon includes multiple virulence-linked factors and their function is crucial for the pathogenicity of all three human pathogenic Yersinia species.Citation32-Citation36 In the enteropathogenic Yersinia species, Y. pseudotuberculosis and Y. enterocolitica, RovA activates expression of invA, which contributes to an efficient colonization of the Peyer’s patches and allows faster progression of the infection.Citation34,Citation35,Citation37 A Y. pestis CO92 ΔrovA mutant is strongly attenuated (≈80-fold by LD50) and colonization of spleen and lung in mice is abolished upon subcutaneous injection, whereas only a slight attenuation was observed when the pathogen was given via an intranasal or intraperitoneal route. This indicated that RovA plays a more important role in bubonic plague than pneumonic plague or systemic infection.Citation33
The rovA locus of Y. pestis and Y. pseudotuberculosis is 100% identical. It is transcribed by two promoters and is positively autoregulated in both pathogens.Citation28,Citation38 Expression of rovA is also strongly thermoregulated. This is based on the fact that RovA is an intrinsic temperature-sensing regulator in which thermally induced conformational changes interfere with DNA-binding capacity, and render RovA susceptible to proteolytic degradation.Citation39 In addition, rovA transcription was shown to be strongly influenced by the nutrient composition of the growth medium, and this is mediated by the Crp-CsrABC-RovM regulatory cascade ().Citation30,Citation35,Citation40
The global regulator Crp regulates uptake and catabolism of carbohydrates and was found to control expression of the two regulatory RNAs CsrB and CsrC in Y. pseudotuberculosis, which are part of the carbon storage regulator (Csr) system.Citation40,Citation41 Both Csr RNAs harbor several GGA motifs, which promote binding/sequestration and inactivation of the global post-transcriptional regulator protein CsrA. Binding of CsrA typically blocks translation initiation of its target mRNAs and this is often accompanied by an accelerated mRNA decay.Citation42,Citation43 The CsrA recognition site seems to be quite variable; however, a highly conserved GGA motif that is often present in the loop of short hairpins was found to be highly conserved.Citation43
Among the CsrA target mRNAs of Yersinia and other related pathogens, are many classical virulence genes and regulators, but also multiple virulence-linked metabolic, physiological (e.g., motility), and stress adaptation traits.Citation40,Citation41,Citation44
In the present work, we studied the influence of PhoP on the complex CsrABC-RovM-RovA regulatory cascade in Y. pseudotuberculosis mediating the expression of important early stage virulence genes such as invasin. We found that PhoP activates csrC expression directly from two different promoters. The stability of the resulting CsrC RNAs differs significantly between the Y. pseudotuberculosis strains YPIII and IP32953, due to a 20 nucleotides insertion in the IP32953 CsrC RNA, which renders the transcript more susceptible to degradation. Our work shows that the regulatory cascade controlling RovA can vary significantly between different Y. pseudotuberculosis isolates, suggesting evolutionary changes that adapt expression of the individual sets of pathogenicity factors to specific niches within hosts.
Results
PhoP activates rovA expression in Y. pseudotuberculosis via RovM
Previous work with Y. pestis demonstrated that the PhoP/PhoQ two-component system influences orthologs of two virulence regulators, Crp and RovA, controlling expression of important early stage virulence genes in the close relative Y. pseudotuberculosis ().Citation28,Citation29 In order to test the role of PhoP in Y. pseudotuberculosis, we first investigated the influence of the response regulator on RovA expression in the wild-type strains YPIII and IP32953. Both strains have most frequently been used to elucidate Y. pseudotuberculosis pathogenicity. However, recently it was found that YPIII carries a mutation in the PhoP-encoding gene YPK_1715, leading to a non-functional version of PhoP.Citation45 Therefore, we first constructed a set of isogenic phoP+ and phoP- derivatives of YPIII and IP32953. To do so, the non-functional phoP gene (phoP-) of YPIII was replaced by the functional phoP+ gene of IP32953, yielding strain YP149 (YPIII phoP+). Furthermore, the functional phoP+ gene of IP32953 was exchanged by the mutated version of YPIII, yielding strain YPIP06 (IP32953 phoP-). Subsequently, RovA levels of the two sets of phoP+ and phoP- strains were analyzed by western blotting at three different growth temperatures, leading to high (25 °C), intermediate (32 °C), and repressed (37 °C) expression of RovA. As shown in , the intracellular amount of RovA differed significantly between the phoP+ and phoP- derivatives of IP32953 and YPIII at 32 °C. At this growth temperature, RovA levels were considerably decreased in both phoP-negative strains. At 25 °C, RovA levels were still markedly reduced in the phoP- derivative of IP32953, whereas similar levels of RovA were observed in the YPIII phoP- and its isogenic phoP+ variant (). RovA was not detectable at 37 °C in any of the tested strains. This strongly indicated that PhoP acts as an activator of RovA expression in Y. pseudotuberculosis at moderate growth temperatures.
Figure 2. PhoP-dependent RovA expression is mediated via RovM. Whole cell extracts of YPIII (phoP-), YP149 (YPIII phoP+), IP32953 (phoP+), and YPIP06 (IP32953 phoP-) grown to exponential phase at 25 °C, 32 °C, and 37 °C were separated by SDS-PAGE prior to western blotting using polyclonal RovA- and RovM-specific antibodies. As negative controls rovA and rovM deletion strains YP107 and YP72 were included. Relative protein amounts were determined densiometrically using the software ImageJ for three independent experiments and normalized to the respective unspecific protein band (c). Statistical analysis was performed by student’s t test with *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.001; n.s., not significant; and n.d., not detectable.
Activation of RovA expression by PhoP could occur directly or indirectly through other regulators, such as RovM, H-NS, or the RovA-targeting proteases Lon and ClpP.Citation35,Citation38,Citation39 We addressed synthesis of the regulators in the phoP+ and phoP- derivatives of YPIII and IP32953 and found that RovM levels were significantly increased in PhoP-deficient strains at 25 °C, 32 °C, and 37 °C (). This strongly indicated that PhoP-dependent regulation of RovA expression in Y. pseudotuberculosis is mediated through downregulation of the transcriptional repressor RovM. Furthermore, we found that RovM levels were generally higher in IP32953 compared with YPIII (), suggesting that not only PhoP, but also other regulatory components contribute to differential RovM levels.
PhoP-dependent expression of RovM is mediated via the small regulatory RNA CsrC
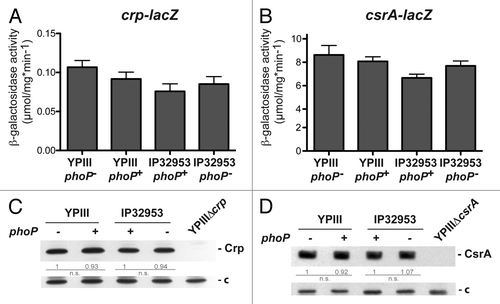
In previous studies we showed that synthesis of the LysR-type regulator RovM is controlled by the global regulator Crp and the Csr system in a nutrient-dependent manner.Citation30,Citation35,Citation40,Citation41 To test whether PhoP-dependent regulation of RovM is linked to Crp and the Csr-system, we monitored expression of crp and csrA using translational lacZ fusions (), and compared the amounts of Crp and CsrA between the phoP+ and phoP- derivatives of YPIII and IP32953. We did not observe any significant influence of PhoP on Crp and CsrA synthesis at 25 °C and 37 °C with both isolates (; Fig. S1).
Figure 3. Analysis of crp and csrA expression in phoP+ and phoP- strains. The vector pAKH139, harboring a crp-lacZ fusion (A) and plasmid pKB63, harboring a csrA-lacZ fusion (B) were transformed into Y. pseudotuberculosis strains YPIII (phoP-), YP149 (YPIII phoP+), IP32953 (phoP+), and YPIP06 (IP32953 phoP-). The bacteria were grown to exponential growth phase in LB medium at 25 °C. The data represent the mean ± SEM from three independent experiments each performed in triplicates. Whole cell extracts of YPIII (phoP-), YP149 (YPIII phoP+), IP32953 (phoP+), and YPIP06 (IP32953 phoP-) grown to exponential growth phase at 25 °C were separated by SDS-PAGE prior to western blotting using polyclonal Crp- (C) and CsrA (D)-specific antibodies. As negative controls crp and csrA deletion strains YP89 and YP53 were included. Relative protein amounts were determined densiometrically using the software ImageJ for three independent experiments and normalized to the respective unspecific protein band (c). Statistical analysis was performed by student’s t test with *, P ≤ 0.05; **, P ≤ 0.005; ***, P ≤ 0.001; n.s., not significant; and n.d., not detectable.
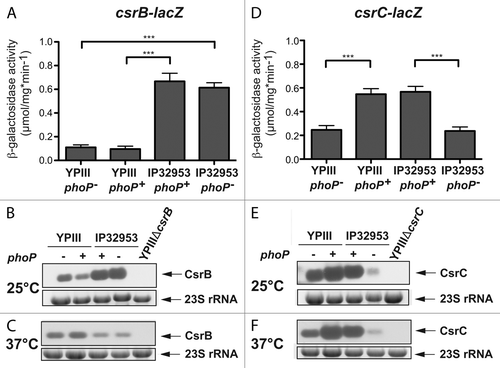
Alternatively, it is possible that PhoP induces an upregulation of the CsrB and/or CsrC RNA, which both sequester and inhibit the function of CsrA, and this would lead, in turn, to a reduction of RovM synthesis ( and ). To determine whether PhoP acts on Y. pseudotuberculosis Csr RNAs, we analyzed expression of csrB- and csrC-lacZ reporter fusions and performed northern blot experiments to compare CsrB and CsrC levels in the phoP+ and phoP- derivatives of YPIII and IP32953 (). In contrast to YPIII, CsrB is thermoregulated in IP32953 and highly expressed at 25 °C, but not at 37 °C (). Furthermore, CsrB synthesis was not affected in both strains by the absence of a functional phoP gene (). In contrast, csrC expression was significantly reduced in both Y. pseudotuberculosis strains lacking a functional phoP gene at 25 °C and 37 °C (). This indicated that the response regulator stimulates csrC transcription. The overall influence of PhoP on CsrC levels seemed much more pronounced in IP32953 as significantly lower amounts of the CsrC transcript were detectable in the phoP-deficient mutant of this isolate.
Figure 4. Influence of PhoP on CsrB and CsrC synthesis. The vector pAKH101, harboring a csrB-lacZ fusion (A), and the vector pAKH103, harboring a csrC-lacZ fusion (D), were transformed into Y. pseudotuberculosis strains YPIII (phoP-), YP149 (YPIII phoP+), IP32953 (phoP+), and YPIP06 (IP32953 phoP-). The cells were grown to exponential growth phase in LB medium at 25 °C. The data represent the mean ± SEM from at least two different experiments each done in triplicate and were analyzed with Student’s t test. ***P < 0,001. Total RNA from Y. pseudotuberculosis strains YPIII (phoP-), YP149 (YPIII phoP+), IP32953 (phoP+), and YPIP06 (IP32953 phoP-) grown to exponential growth phase at 25 °C (B and E) or 37 °C (C and F) was prepared and analyzed by northern blotting with CsrB- (B and C) and CsrC (E and F)-specific probes. YPIII ΔcsrB and YPIII ΔcsrC served as negative control, respectively.
PhoP interacts with the csrC regulatory upstream region
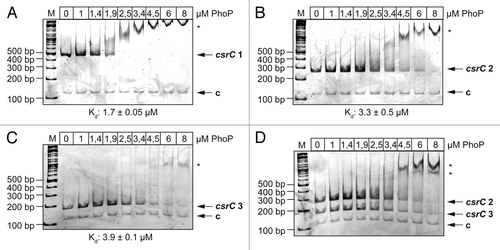
In silico analysis identified two putative PhoP binding sites in the csrC regulatory region. One PhoP binding motif (position 61013-61030 of YPIII; NC_010465) is located 32 nucleotides upstream of the identified transcriptional start site (TSS) of csrC (position 61063).Citation40 The second putative binding site is located more upstream at position 60951-60968, i.e., 94 nucleotides upstream of the transcriptional start site. Gel retardation assays were performed with increasing amounts of purified His-tagged PhoP protein to test whether PhoP is able to interact directly and specifically with the csrC regulatory region. DNA fragments encompassing different portions of the csrC upstream region and a control fragment encoding the gyrA gene were incubated with increasing concentrations of PhoP protein and assayed for protein-DNA complex formation. As shown in , the PhoP protein interacted strongly in a dose-dependent manner with the csrC regulatory region containing both putative PhoP binding sites (-297 to +93), but no binding was detectable to the gyrA control fragment. We further tested if PhoP binds to DNA fragments containing only one of two predicted binding sites. PhoP interacted specifically and with a similar affinity with both fragments (-297 to -55 and -76 to +93, relative to the transcriptional start site at position 61063) (). This observation was confirmed by a competitive gel retardation assay, including both fragments of the csrC regulatory region with single binding sites ().
Figure 5. Interaction of PhoP with the csrC promoter region. Three different DNA promoter fragments of csrC were incubated with increasing concentrations of PhoP. A gyrA fragment was used as negative control (c). The PhoP–DNA complexes were separated on 5% polyacrylamide gels. The position of the specific higher molecular weight DNA–protein complexes is marked with an asterisk. The analyzed fragments were the following: csrC 1 (-297 to +93) (A), csrC 2 (-297 to -55) (B), csrC 3 (-76 to +93) (C), and csrC 2 (-297–-55) competitive with csrC 3 (-76–+93) (D). The relative amount of unbound DNA was determined densiometrically using the software ImageJ and the dissociation constant (Kd) was calculated. The Kd values represent the mean ± SEM of three independent experiments.
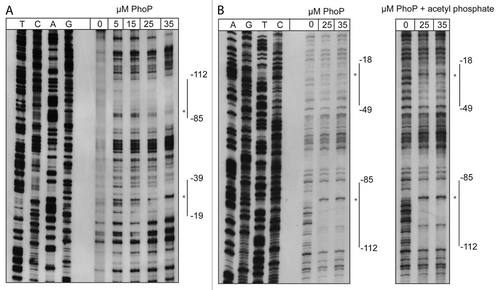
To define the precise binding sites, DNase I footprinting experiments were performed. The purified recombinant His-tagged PhoP protein protected two distinct regions within the entire csrC regulatory region (). The analysis of the antisense and sense strand revealed two footprints, extending from -112 to -85 and -39 to -19 for the antisense strand, and from -18 to -49 and -85 to -112 for the sense strand, overlapping the predicted binding sites. Binding of PhoP to the site closely upstream of the TSS was less efficient, but could be improved by preincubation of purified PhoP with acetyl phosphate. Within the protected regions, two nucleotides became hypersensitive toward DNase I in the presence of higher PhoP concentrations (). In summary, our data show that PhoP interacts directly and specifically with two distinct sites located within the csrC regulatory region to stimulate csrC transcription.
Figure 6. DNase I footprinting of PhoP with the csrC regulatory region. Sense (A) and antisense (B) csrC-probes were DIG-labeled and incubated with increasing amounts of purified His-PhoP or His-PhoP preincubated with acetyl phosphate. DNA or PhoP–DNA complexes were digested with DNase I and separated on a 6% polyacrylamide gel. Lanes T, C, A, and G represent the Sanger sequencing reactions. The DNase I protected regions are indicated with vertical bars, hypersensitive regions are marked with an asterisk. The numbers indicate the nucleotide positions upstream of csrC.
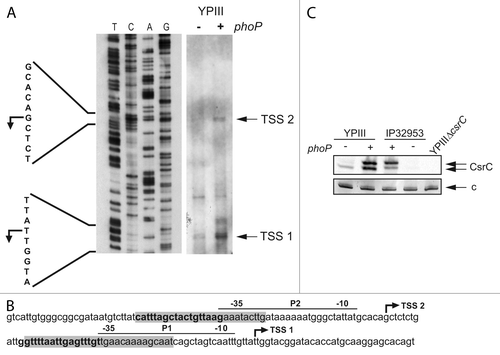
PhoP-dependent expression of csrC is initiated from two transcriptional start sites
Next, we analyzed whether PhoP-dependent csrC expression is initiated from the promoter previously identified in our studies.Citation40 We mapped the 5′-ends of the CsrC RNA and compared the amount of primer extension products in the phoP+ and phoP- YPIII strain (). We selected YPIII for this analysis as in contrast to IP32953 CsrC was still detectable in the absence of a functional phoP gene (). One transcriptional start site (TSS 1) was mapped in both derivatives of YPIII, whereby the transcription level from this site was much more enhanced in the YPIII phoP+ derivative (). This start site is identical with the transcriptional start site identified earlier at strand position 61063.Citation40 Most strikingly, an additional start site (TSS 2) was located 61 nucleotides further upstream of TSS 1, which was more active in the YPIII phoP+ derivative (YP149). A putative promoter sequence was identified closely upstream of both transcriptional start sites, and each of the -35 regions of the promoters was flanked by one PhoP box (). This indicates that PhoP activates expression of CsrC from two distinct promoters, leading to a shorter and an extended CsrC transcript. To verify the production of different CsrC species in the presence of PhoP, we separated total RNA of the phoP+ and phoP- derivatives of YPIII and IP32953 on polyacrylamide gels to separate the predicted transcripts and performed northern blot experiments. Two CsrC RNA species were detectable in both Y. pseudotuberculosis strains in the presence of PhoP, but only the shorter CsrC transcript was visible in YPIII harboring the non-functional PhoP allele ().
Figure 7. PhoP-dependent transcription of csrC occurs from two distinct start sites. (A) Mapping of the csrC transcriptional start site. For primer extension of the CsrC transcript total RNA isolated from YPIII and YP149 (YPIII phoP+) grown at 25 °C in LB, and a Dig-labeled csrC-specific primer was used. The sequencing reaction (left site) was performed with the same primer used for the extension reaction. The transcriptional start sites (TSSs) are indicated by arrows. (B) Regulatory region of csrC, illustrating the two putative promoters, the identified transcriptional start sites, the DNase I protected areas (highlighted in gray), and putative PhoP boxes (bold letters). (C) High-resolution northern blot of CsrC revealing two CsrC isoforms. Total RNA was isolated from YPIII (phoP-) and YP149 (YPIII phoP+) as well as IP32953 (phoP+) and YPIP06 (IP32953 phoP-) grown to exponential growth phase at 25 °C in LB. Ten µg of RNA were separated using urea-acrylamide gels and analyzed by northern blotting with CsrC-specific probes. YPIII ΔcsrC served as negative control.
Different stability of the CsrC transcripts in YPIII and IP32953
Our comparative analysis of CsrC levels in this study has shown that the CsrC RNA was much less abundant in the phoP- derivative of IP32953 compared with YPIII harboring the identical phoP mutation (). Since the transcription of the csrC-lacZ fusion did not differ between the strains (), we hypothesized that the stability of the CsrC transcripts is reduced in IP32953.
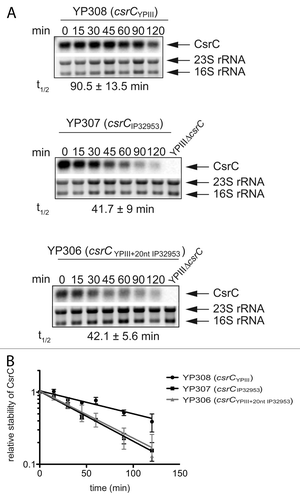
Overall, the csrC locus is highly conserved between the Y. pseudotuberculosis strains YPIII and IP32953. However, an additional 20 nucleotides stretch is present within the csrC gene of IP32953 that is absent in YPIII and in Y. pestis CO92 (Fig. S2). Based on the repetitive DNA sequence, it is likely that this variation is the result of two separate duplication events. To address whether this insertion has an influence on CsrC stability, we first determined the stability of CsrCYPIII and CsrCIP32953 in strain YP285, harboring a functional phoP gene ensuring maximal expression of the CsrC RNAs. As shown in , the CsrC RNA of YPIII was slowly degraded with a half-life of about 90 min, whereas CsrC of IP32953 was less stable and decayed with a half-life of about 42 min. Furthermore, we integrated the additional 20 nucleotides stretch of the IP32953 CsrC RNA into CsrC of YPIII and tested the stability of the mutated transcript in the YPIII phoP+ background (). Degradation of the modified CsrC transcript was considerably enhanced. It decayed with a half-life similar to the IP32953 CsrC RNA, indicating that the insertion within CsrC promotes a more rapid degradation of this regulatory RNA in the Y. pseudotuberculosis wild-type strain IP32953.
Figure 8. RNA stability assay of CsrC. (A) A RNA stability assay of CsrC in the three different strains YP308 (CsrCYPIII), YP307 (CsrCIP32953), and YP306 (CsrCYPIII+20nt IP32953) was performed. RNA synthesis was stopped by adding 2 mg/ml rifampicin and samples were taken after 0, 15, 30, 45, 60, 90, and 120 min. (B) The half-life of CsrC was measured by a least squares analysis of semi-logarithmic plots of RNA concentration vs. time and represent the mean ± SEM from three independent experiments.
Discussion
The ability of pathogenic bacteria to adjust their virulence-associated traits, physiological features, and metabolic properties in response to the rapidly changing conditions within the host is crucial for a successful infection. Recently, it became evident that many invading bacteria use two-component systems to monitor nutritional, ion, and physical changes and reprogram the expression of small regulatory RNAs of the post-transcriptional Csr/Rsm systems to manage virulence.Citation41,Citation44 In the related species E. coli and Salmonella, both Csr RNAs (CsrB and CsrC) are activated by the BarA/UvrY system at low pH and in the presence of weak organic acids.Citation46,Citation47 Moreover, all known Csr-type RNAs of Legionella pneumophila, Pseudomonas aeruginosa, and Vibrio cholerae are induced by homologs of the BarA/UvrY system (LetS/LetA, VarS/VarA, and GacS/GacA). In Yersinia, only csrC is activated by BarA/UvrY,Citation30,Citation40 whereas transcriptional regulators of csrC remained unknown.
In the present study, we show that the Y. pseudotuberculosis Csr system is also strongly regulated by the response regulator PhoP ( and ). Notably, an implication of the global Mg2+-responsive two-component system PhoP/PhoQ in the control of other Csr/Rsm systems has not previously been reported. PhoP induces transcription of the csrC gene, but not of csrB, by binding to two distinct PhoP box-like sequences within the csrC regulatory region. PhoP-dependent transcription of csrC starts from two independent start sites identified in close proximity of the identified PhoP binding sites. As a result, two small regulatory RNA species that differ 61 nucleotides in length are produced in the presence of PhoP, but not in the phoP-deficient strains. Whether the different transcripts vary in their function, e.g., in their capacity to bind CsrA is unclear. However, no additional potential CsrA binding sites (GGA motifs) are present in the added 5′-end of the extended CsrC transcript (Fig. S2). The formation of an additional hairpin structure, but no major changes of the overall CsrC structure were observed between the two variants as predicted by the Mfold softwareCitation48 (Fig. S3).
Figure 9. Model of PhoP-dependent regulation of csrC transcription. The current model displays PhoP-dependent regulation of csrC expression and derivative-specific differences in CsrC transcript stability in Y. pseudotuberculosis YPIII and IP32953. Expression of csrC is positively regulated by PhoP via direct DNA-interaction. PhoP stimulates csrC expression from two distinct promoters resulting in two CsrC isoforms. IP32953 CsrC carries an additional 20 nucleotides stretch (black box) leading to decreased CsrC stability.
The molecular nature of the environmental stimuli controlling the Csr/Rsm systems in pathogens during infection is mostly unknown. However, a range of distinct incoming signals and participating regulators has been identified leading to a differential expression of the Csr-type RNAs. In P. aeruginosa, expression of both Rsm RNAs RsmY and RsmZ is differentially regulated through multiple sensor kinases and signaling pathways that converge to the GacA response regulator and control both sRNAs, or induce only the production of RsmY.Citation49 Moreover, the RsmY sRNA is more abundant than RsmZ, as the global H-NS family regulators MvaT and MvaU bind to an AT-rich motif in the upstream region of rsmZ to repress transcription, whereas rsmY is not affected.Citation50,Citation51 In Y. pseudotuberculosis, the initial input comes from the independent two-component systems BarA/UvrY and PhoP/PhoQ, as well as Crp, which adjust the final output by a differential control of CsrB and CsrC levels according to the availability of nutrients and ions. The PhoP/PhoQ system responds to low Mg2+ and acidic pH frequently found within macrophages, and to various host-derived antimicrobial peptides that are part of the native immune response.Citation52 BarA/UvrY and Crp enable the bacteria to adjust to a switch of favored catabolites (e.g., glucose) and an imbalance of TCA cycle intermediates (e.g., acetate, formate).Citation30,Citation46,Citation47 These features underpin the possibility to fine-tune regulation of virulence determinants required to survive conditions experienced during extracellular and intracellular growth. There is evidence that Y. pseudotuberculosis is predominantly localized in extracellular sites during a systemic infection.Citation53 However, the bacteria can also multiply intracellularly in professional phagocytes (e.g., macrophages).Citation45,Citation54 This process is believed to be particularly important during the early stages of a systemic infection, in which the phagocytes appears to act as a trojan horse to reach deeper tissues. The phagocytes also function as a shelter for the pathogens to proliferate and induce pathogenicity determinants that enable them to annihilate the host immune response.
On the basis of this knowledge, it is likely that differences in the genetic equipment of the individual regulatory systems in different isolates of Y. pseudotuberculosis or in the closely related descendant Y. pestis determine distinct virulence properties. Here, we demonstrate that the expression of the Csr-type RNAs differs largely between the two Y. pseudotuberculosis isolates YPIII and IP32953. One reason is that csrC transcription in YPIII is reduced due to the absence of a functional phoP gene. Additionally, the stabilities of the CsrC RNAs are very different between the two Y. pseudotuberculosis strains, due to a 20 nucleotide insertion in the IP32953 CsrC RNA rendering the transcript much more susceptible to degradation (). As a result, CsrC levels are significantly lower in a phoP- derivative of Y. pseudotuberculosis IP32953 compared with strain YPIII lacking a functional phoP allele. Differential regulation of Csr-type RNAs within Y. pseudotuberculosis is further supported by our finding that csrB expression is temperature-regulated in IP32953, but not in YPIII. The molecular basis for the different csrB expression pattern is still unknown, but it does not seem to require PhoP.
The different abundance of the Csr RNAs in YPIII and IP32953 contribute to varying levels of the virulence regulator RovA, inducing the expression of several virulence-associated traits, including the colonization factors InvA and PsaA.Citation32,Citation33 In YPIII, RovA is strongly expressed at moderate temperatures (25 °C) despite the absence of PhoP, whereas in IP32953, PhoP is required to fully induce RovA expression. It seems feasible that enhanced RovA expression due to a stable CsrC RNA in YPIII allowed loss of a functional phoP gene, while the instable CsrC variant in IP32953 possibly demands presence of a functional phoP copy. A recent study revealed that PhoP also influences rovA expression in Y. pestis.Citation28 However, the regulation patterns seem considerably different from that observed in Y. pseudotuberculosis, which might indicate interesting evolutionary changes in the regulation of crucial virulence factors between these species. Zhang, et al. reported that PhoP inhibits transcription of rovA under Mg2+-limiting conditions, but has no influence under Mg2+-rich or acidic pH conditions.Citation28 Under inducing conditions, PhoP recognizes a single site within the rovA regulatory region overlapping the transcriptional start site of promoter P1.Citation28 Moreover, Y. pestis PhoP was shown to activate crp expression in rich medium.Citation29 However, in contrast to Y. pestis, PhoP does not affect crp expression and Crp levels in Y. pseudotuberculosis when cultured in rich medium. The species-specific differences are surprising, since the crp, csrB, csrC, and the rovA promoter regions are highly conserved between Y. pestis and Y. pseudotuberculosis,Citation55,Citation56 indicating that different regulator variants or other regulatory elements participate in the control of the Csr system in different yersiniae. This clearly illustrates that the regulatory networks controlled by the global regulators PhoP, Crp, and CsrABC are tightly interwoven in order to control metabolism, host/environmental adaptation, and virulence in the genus Yersinia. However, the regulatory circuits and contributing components seem to vary greatly within and among Yersinia species, suggesting that the Csr system is a focal point of global control to adapt the genus to different hosts and reservoirs.
In this context, it would also be interesting to know how the different outcomes of the PhoP-CsrABC-RovM-RovA regulatory cascade affect the infection process in the different Yersinia strains and species. Several studies demonstrated that the PhoP/PhoQ system is important for Yersinia virulence. It was shown in in vitro studies that Y. pseudotuberculosis and Y. pestis strains lacking the response regulator PhoP display an impaired survival and replication capacity inside macrophages and/or neutrophils.Citation22,Citation45,Citation57,Citation58 The role of PhoP for the virulence of both Yersinia species was also investigated by subcutaneous, aerosol, and oral challenges of mice. However, the overall defect of the individual phoP mutants varied significantly between different isolates of the two species. For instance, a 75-fold higher LD50 has been determined for the Y. pestis strain GB mutated in phoP by subcutaneous injections of mice (bubonic model),Citation22 whereas only a modest defect was seen for a phoP mutant of Y. pestis CO92.Citation24 Furthermore, PhoP does not seem to play a role for virulence of Y. pestis CO92 after an aerosol challenge (pneumonic model),Citation24 but it is important for Y. pseudotuberculosis IP32953 and IP2666 during aerosol infections.Citation23,Citation24 How PhoP and different levels of the Csr RNAs affect virulence of YPIII and IP32953 after a natural oral challenge of mice is currently unknown. One report describes that YPIII has a reduced ability to colonize lungs in a systemic model in comparison to the Y. pseudotuberculosis phoP+ strain IP2666.Citation23 However, due to observed strain- and species-specific differences a more detailled analysis comparing the regulatory circuits of PhoP and the Csr regulon in different Y. pestis and Y. pseudotuberculosis strains with their impact on virulence is required to fully understand how this regulatory node adapts individual yersiniae to different reservoirs and host niches.
Materials and Methods
Bacterial strains, media, and growth conditions
The strains used in this work are listed in . Bacteria were routinely grown in Luria-Bertani (LB) broth to exponential growth phase (OD600nm = 0.4–0.6) at 25 °C, 32 °C, or 37 °C under aerobic conditions. If necessary, antibiotics were added at the following concentrations: ampicillin 100 µg ml−1, chloramphenicol 30 µg ml−1, and kanamycin 50 µg ml−1.
Table 1. Bacterial strains and plasmids
DNA manipulation and plasmid construction analysis
All DNA manipulations, restriction digestions, ligations, and transformations were performed using standard genetic and molecular techniques.Citation59,Citation60 The plasmids used in this work are listed in . Oligonucleotides used for PCR, sequencing, and primer extension were purchased from Metabion and are listed in . Plasmid DNA was isolated using Qiagen plasmid preparation kits. DNA-modifying enzymes and restriction enzymes were purchased from Roche or New England Biolabs. PCRs were done in a 100 µl mix for 29 cycles using Taq polymerase (Promega) or Phusion High-Fidelity DNA polymerase (New England Biolabs). Purification of PCR products was routinely performed using the QIAquick kit (Qiagen) or the Nucleic Acid and Protein Purification kit (Macherey Nagel). All constructed plasmids were sequenced by the in-house facility or GATC.
Table 2. Oligonucleotides
To generate plasmid pAKH103, the upstream regulatory region of csrC (-355 to +4) was amplified using primers 774 and I363 and cloned into the EcoRI/SalI sites of pHT124. Plasmid pAKH139 carries a PCR-generated fragment harboring the crp promoter region from nucleotide -491 (primer II432) to nucleotide +3 (primer II433) relative to the translational start. The fragment was digested and inserted into the XhoI/SalI site of pFU67 resulting in a translational crp-lacZ fusion. The suicide mutagenesis plasmid pAKH188 was constructed by insertion of the additional 20 nucleotides of csrC of IP32953 into the csrC chromosomal sequence of YPIII. To do so, QuickChange mutagenesis (Stratagene) of pFS31 was performed using primers V604 and V605 according to the manufacturer’s instructions. For the construction of plasmid pFS13, the entire phoP coding region (+1 to +671) was amplified from Y. pseudotuberculosis IP32953 with primers IV703 and IV704 and cloned into the BamHI and HindIII sites of plasmid pET28a (Novagen). To generate plasmids pFS29 and pFS31, the csrC+ fragments of Y. pseudotuberculosis strains IP32953 and YPIII were amplified by PCR using primers II850 and II853, digested with SacI, and inserted into the suicide mutagenesis plasmid pAKH3. To monitor csrA transcription, the csrA promoter region (-1071) and 16 nucleotides of the coding region were amplified by PCR using primers I617 and II275. The amplified fragment was subsequently inserted into the PstI site of pTS02, resulting in pKB63. For the construction of plasmid pVP3, a kanamycin resistance cassette with 500 bp flanking regions of the phoPQ locus was amplified with primer pair III925/III926 from chromosomal DNA of YP56 and ligated into the SacI/XhoI sites of pDM4. Plasmid pWH1 was constructed by amplification of phoPQ of Y. pseudotuberculosis IP32953 with its 500 bp flanking regions using the primers III926 and III927. The fragment was ligated into the SacI/XhoI sites of pDM4.
Construction of a csrC-deficient derivative of the YPIII phoPQ+ strain YP149Citation61 was performed by homologous recombination using suicide plasmid pAKH149 as described previously.Citation30 The resulting strain YP285 was used for generation of strains YP306, YP307, and YP308. The suicide plasmids pAKH188, pFS29, and pFS31 were mated from S17-1λpir (tra+) into YP285. Transconjugants were selected by plating on Yersinia selective agar (Oxoid) supplemented with ampicillin. Subsequently, the resulting strains were plated on LB plates containing 10% sucrose, which induces the expression of the sacB gene on the integrated plasmid, leading to the production of a toxic, growth-reducing substance. Fast growing colonies were selected to identify clones in which the integrated plasmid, including the wild-type copy of the target gene, was lost by a second spontaneous recombination process. The resulting strains were tested for ampicillin sensitivity and presence of the mutations was analyzed by PCR and sequencing.
In order to generate Y. pseudotuberculosis IP32953 ΔphoPQ strain YPIP04, pVP3 was integrated into the phoPQ locus of strain IP32953 via conjugation as described previously.Citation34 Chromosomal integration of the fragment was selected by plating the bacteria on Yersinia selective agar supplemented with kanamycin. Conjugants with an excision of the plasmid including the functional phoPQ operon of IP32953 were identified after plating on 10% sucrose and selection of fast-growing, chloramphenicol-sensitive strains as described above. Absence of the phoPQ operon was tested by PCR. To construct Y. pseudotuberculosis IP32953 phoP- strain YPIP06 harboring the non-functional phoP gene of YPIII, the plasmid pWH1 was conjugated into YPIP04. Chromosomal integration of the plasmid was selected by plating on Yersinia selective agar supplemented with chloramphenicol. The correct mutant was identified after plating on 10% sucrose as following: (1) fast-growing bacteria were isolated and tested for loss of the kanamycin cassette, (2) presence of the phoPQ operon of YPIII was analyzed by PCR with primers III964, III965, III966, and III967, and (3) presence of the phoPQ, including the non-functional allele of phoP, was confirmed by PCR and sequencing.
Expression and purification of the PhoP protein
E. coli strain BL21λDE3 pFS13 was grown at 37 °C to exponential growth phase (OD600nm = 0.4–0.6). Subsequently, the bacteria were shifted to 18 °C and expression of His-tagged PhoP (His6-PhoP) was induced with 1 mM IPTG (isopropyl-β-D-thiogalactoside). After 3 h, bacteria were harvested by centrifugation (4 °C, 20 min, 6.000–9.000 g) and resuspended in lysis buffer (50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazol, pH 8.0). Bacterial cells were lysed with a French Press (Heinemann) and soluble His6–PhoP protein was purified using a Ni-NTA agarose column (Macherey Nagel). The column was washed with four column volumes washing buffer (50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazol, pH 8.0) and the His-tagged PhoP protein was eluted with elution buffer (50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazol, pH 8.0). The purity of PhoP (> 95%) was verified by SDS-PAGE.
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as described previouslyCitation35 with some modifications. For DNA-binding studies, three different fragments of the csrC upstream region were amplified by PCR from genomic DNA of Y. pseudotuberculosis YPIII. One fragment, including both putative PhoP binding sites, was amplified with primer pairs IV937/IV939 (). The other fragments harboring either PhoP binding site 1 or 2 were amplified with primer pairs IV938/IV939 and IV937/IV940, respectively. A control fragment containing a part of the gyrA gene was amplified with primers III186/III187. The purified PhoP protein was dialyzed against DNA-binding buffer (20 mM NaH2PO4, 100 mM KCl, 5% glycerol, 2 mM 1,4-dithiothreitol, pH 8.0). Equimolar ratios of the DNA fragments and increasing amounts of PhoP were incubated in a 20 µl reaction mixture for 20 min at 25 °C in DNA-binding buffer. The reaction mixture was immediately loaded and separated on a 5% polyacrylamide gel, and stained with ethidium bromide. The relative amount of unbound DNA was determined densiometrically using the software ImageJ (http://imagej.nih.gov/ij/) and the dissociation constant (Kd) was calculated. The Kd values represent the mean ± SEM of three independent experiments.
DNase I footprinting
The DNase I footprinting experiments were performed as describedCitation35 with the following modifications. First, the csrC promoter region was amplified from Y. pseudotuberculosis YPIII using a sense digoxigenin (DIG)-labeled primer (I293) and a non-labeled primer (I79), or an anti-sense digoxigenin (DIG)-labeled primer (V586) and a non-labeled primer (V587). Either the His-tagged PhoP was used directly for the footprint assay or the PhoP protein was phosphorylated by preincubation with 20 mM acetyl phosphate for 30 min at 25 °C. The purified PCR product was incubated with increasing amounts of His-tagged PhoP protein for 20 min in DNA-binding buffer as described for the EMSA. The samples were treated with an appropriate dilution of DNase I and stopped after 20 s by adding 50 µl stop solution (15 mM EDTA, 10 µg ml−1 yeast carrier tRNA). The DNA was extracted by adding phenol-chloroform-isoamylalcohol (25:24:1) and precipitated with ethanol. The samples were loaded on a 6% polyacrylamide sequencing gel and run for 3 h at 60 W. The digested DNA fragments were transferred on a Nytran membrane (GE Healthcare), UV crosslinked, and the protected DNA bands were detected using CDP-Star according to the DIG Luminescent Detection kit (Roche). The DNA fragments used for the footprint reaction were generated with the same DIG-labeled primer used for the amplification of csrC for the sequencing reaction.
The sequencing reaction of the csrC upstream region was performed using the dideoxy-chain reaction with the Thermo Sequenase cycle sequencing kit (Affymetrix) according to the manufacturer’s instructions. The csrC upstream region was amplified from the plasmid pAKH59 with the digoxigenin (DIG)-labeled sense primer I293 or the anti-sense primer V586.
Primer extension analysis
Primer extension analysis was performed as described previouslyCitation35 with minor modifications. Total RNA was isolated using the hot phenol method and 20 µg of total RNA were reverse transcribed using the digoxigenin-labeled primer I293 specific for the csrC gene. The sequencing reaction was performed with primer I293 as described for DNase I footprinting.
Western blotting
For the detection of the regulatory proteins RovA, RovM, Crp, and CsrA, cultures of the Y. pseudotuberculosis strains were grown under specific environmental conditions as described. Bacterial whole cell extracts were prepared from equal amounts of bacteria and separated on SDS-polyacrylamide gels, and blotted onto nitrocellulose membranes.Citation60 Subsequently, membranes were blocked in 1 × TBST containing 3% BSA (blocking buffer). Primary, polyclonal rabbit IgG antibodies (anti-RovA, anti-RovM, anti-Crp, and anti-CsrA) were added in a 1:4 000 dilution in blocking buffer. The secondary antibody, anti-rabbit IgG conjugated with horse radish peroxidase, was supplied in a 1:8 000 dilution in blocking buffer and the immunological detection of the proteins was performed as described previously.Citation30,Citation40 Relative protein amounts were determined densiometrically using the software ImageJ (http://imagej.nih.gov/ij/) for three independent experiments and normalized to the respective unspecific protein band. Statistical analysis was performed by student’s t test.
RNA isolation, northern blotting, and RNA stability assays
For the isolation of total RNA, to exponential phase Y. pseudotuberculosis strains were cultivated at 25 °C or 37 °C. Bacterial cells were harvested by centrifugation (10 000 g) at the same temperature as the respective experiment was performed, and the cell pellet was snap-frozen in liquid nitrogen. Total RNA was isolated by the hot phenol method,Citation30 and quantified photometrically using a wavelength of 260 nm.
For northern blotting, total RNA (5 µg) was mixed with loading buffer (0.03% bromophenol blue, 4 mM EDTA, 0.1 mg/ml EtBr, 2.7% formaldehyde, 31% formamide, 20% glycerol in 4 × MOPS buffer), heated for 10 min at 70 °C, separated on MOPS agarose gels (1.2%), and transferred by vacuum blotting for 1.5 h onto positively charged membranes (GE Healthcare) in 10 × SSC. To detect the two different transcripts of CsrC high-resolution gels (urea acrylamide, 12%) were performed. Total RNA (10 µg) was separated for 3 h at 140 V and transferred onto positively charged membranes (GE Healthcare) in 0.5 × TBE for 30 min at 20 V using a semi-dry blotting system. The membrane was UV cross-linked. DIG-labeled csrB and csrC PCR-fragments were amplified with primer pairs 555/556 and 582/583 respectively () using the DIG-PCR nucleotide mix (Roche). Prehybridization (1–2 h, 42 °C), hybridization (overnight, 42 °C), and washing were conducted using the DIG Luminescent Detection kit (Roche) according to the manufactures instructions.
RNA stability assay was used to compare degradation of the different CsrC RNAs. Exponentially grown cultures of bacteria expressing the different CsrC variants were mixed with rifampicin (2 mg/ml) to inhibit transcription. At certain time points after blockage of transcription, samples were withdrawn, mixed with 0.2 volume of stop solution (5% water-saturated phenol, 95% ethanol), and snap-frozen in liquid nitrogen. The pellets were thawed on ice, centrifugated (4 °C, 10 min, 14 000 rpm), and RNA was isolated using the SV total RNA purification kit (Promega) as described by the manufacturer. Separation and detection of the RNA were performed as described above. The half-life of CsrC was calculated by least squares analysis of semi-logarithmic plots of CsrC RNA amounts, normalized to 23S and 16S rRNA, vs. time.
Analysis of reporter gene expression
The β-galactosidase activity of the lacZ fusion constructs was measured in permeabilized cells as described previously.Citation34 The activity was calculated as follows: β-galactosidase activity OD420nm * 6648−1 * OD600 nm−1 * t (min)−1 * Vol (ml)−1.
Additional material
Download Zip (2.3 MB)Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Dr Martin Fenner for helpful and motivating discussions. We also thank Katja Böhme and Verena Pianka for plasmids, and Tanja Krause, Sandra Stengel, and Karin Paduch for experimental support. This work was supported by the Deutsche Forschungsgemeinschaft Grants DE616/3-2 (SPP1258) for Dersch P and Heroven AK, DE616/4-2 (SPP1316) for Nuss AM, young investigator start up funding for Nuss AM (SPP1617), and DE616/5-1 (SPP1617) for Schuster F, a stipend of the HZI Graduate School for Heine W, the Deutsche Zentrum für Infektionsforschung (DZIF) for Pisano F and Dersch P, and the Fonds der Chemischen Industrie for Dersch P.
References
- Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem 2000; 69:183 - 215; http://dx.doi.org/10.1146/annurev.biochem.69.1.183; PMID: 10966457
- Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol 2009; 63:133 - 54; http://dx.doi.org/10.1146/annurev.micro.091208.073214; PMID: 19575571
- Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev 2006; 70:910 - 38; http://dx.doi.org/10.1128/MMBR.00020-06; PMID: 17158704
- Groisman EA. The pleiotropic two-component regulatory system PhoP-PhoQ. J Bacteriol 2001; 183:1835 - 42; http://dx.doi.org/10.1128/JB.183.6.1835-1842.2001; PMID: 11222580
- Gooderham WJ, Hancock RE. Regulation of virulence and antibiotic resistance by two-component regulatory systems in Pseudomonas aeruginosa.. FEMS Microbiol Rev 2009; 33:279 - 94; http://dx.doi.org/10.1111/j.1574-6976.2008.00135.x; PMID: 19243444
- Brodsky IE, Gunn JS. A bacterial sensory system that activates resistance to innate immune defenses: potential targets for antimicrobial therapeutics. Mol Interv 2005; 5:335 - 7; http://dx.doi.org/10.1124/mi.5.6.4; PMID: 16394247
- Kato A, Tanabe H, Utsumi R. Molecular characterization of the PhoP-PhoQ two-component system in Escherichia coli K-12: identification of extracellular Mg2+-responsive promoters. J Bacteriol 1999; 181:5516 - 20; PMID: 10464230
- Soncini FC, Véscovi EG, Groisman EA. Transcriptional autoregulation of the Salmonella typhimurium phoPQ operon. J Bacteriol 1995; 177:4364 - 71; PMID: 7543474
- Li Y, Gao H, Qin L, Li B, Han Y, Guo Z, Song Y, Zhai J, Du Z, Wang X, et al. Identification and characterization of PhoP regulon members in Yersinia pestis biovar Microtus. BMC Genomics 2008; 9:143; http://dx.doi.org/10.1186/1471-2164-9-143; PMID: 18366809
- Minagawa S, Ogasawara H, Kato A, Yamamoto K, Eguchi Y, Oshima T, Mori H, Ishihama A, Utsumi R. Identification and molecular characterization of the Mg2+ stimulon of Escherichia coli.. J Bacteriol 2003; 185:3696 - 702; http://dx.doi.org/10.1128/JB.185.13.3696-3702.2003; PMID: 12813061
- Yamamoto K, Ogasawara H, Fujita N, Utsumi R, Ishihama A. Novel mode of transcription regulation of divergently overlapping promoters by PhoP, the regulator of two-component system sensing external magnesium availability. Mol Microbiol 2002; 45:423 - 38; http://dx.doi.org/10.1046/j.1365-2958.2002.03017.x; PMID: 12123454
- Lejona S, Aguirre A, Cabeza ML, García Véscovi E, Soncini FC. Molecular characterization of the Mg2+-responsive PhoP-PhoQ regulon in Salmonella enterica.. J Bacteriol 2003; 185:6287 - 94; http://dx.doi.org/10.1128/JB.185.21.6287-6294.2003; PMID: 14563863
- Fields PI, Swanson RV, Haidaris CG, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA 1986; 83:5189 - 93; http://dx.doi.org/10.1073/pnas.83.14.5189; PMID: 3523484
- Miller SI, Kukral AM, Mekalanos JJ. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA 1989; 86:5054 - 8; http://dx.doi.org/10.1073/pnas.86.13.5054; PMID: 2544889
- Oyston PC, Dorrell N, Williams K, Li SR, Green M, Titball RW, Wren BW. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis.. Infect Immun 2000; 68:3419 - 25; http://dx.doi.org/10.1128/IAI.68.6.3419-3425.2000; PMID: 10816493
- Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun 2006; 74:3727 - 41; http://dx.doi.org/10.1128/IAI.00255-06; PMID: 16790745
- O’Loughlin JL, Spinner JL, Minnich SA, Kobayashi SD. Yersinia pestis two-component gene regulatory systems promote survival in human neutrophils. Infect Immun 2010; 78:773 - 82; http://dx.doi.org/10.1128/IAI.00718-09; PMID: 19933831
- Hitchen PG, Prior JL, Oyston PC, Panico M, Wren BW, Titball RW, Morris HR, Dell A. Structural characterization of lipo-oligosaccharide (LOS) from Yersinia pestis: regulation of LOS structure by the PhoPQ system. Mol Microbiol 2002; 44:1637 - 50; http://dx.doi.org/10.1046/j.1365-2958.2002.02990.x; PMID: 12067350
- Fields PI, Groisman EA, Heffron F. A Salmonella locus that controls resistance to microbicidal proteins from phagocytic cells. Science 1989; 243:1059 - 62; http://dx.doi.org/10.1126/science.2646710; PMID: 2646710
- Galán JE, Curtiss R 3rd. Virulence and vaccine potential of phoP mutants of Salmonella typhimurium.. Microb Pathog 1989; 6:433 - 43; http://dx.doi.org/10.1016/0882-4010(89)90085-5; PMID: 2671582
- Bozue J, Mou S, Moody KL, Cote CK, Trevino S, Fritz D, Worsham P. The role of the phoPQ operon in the pathogenesis of the fully virulent CO92 strain of Yersinia pestis and the IP32953 strain of Yersinia pseudotuberculosis.. Microb Pathog 2011; 50:314 - 21; http://dx.doi.org/10.1016/j.micpath.2011.02.005; PMID: 21320584
- Oyston PC, Dorrell N, Williams K, Li SR, Green M, Titball RW, Wren BW. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis.. Infect Immun 2000; 68:3419 - 25; http://dx.doi.org/10.1128/IAI.68.6.3419-3425.2000; PMID: 10816493
- Fisher ML, Castillo C, Mecsas J. Intranasal inoculation of mice with Yersinia pseudotuberculosis causes a lethal lung infection that is dependent on Yersinia outer proteins and PhoP. Infect Immun 2007; 75:429 - 42; http://dx.doi.org/10.1128/IAI.01287-06; PMID: 17074849
- Bozue J, Mou S, Moody KL, Cote CK, Trevino S, Fritz D, Worsham P. The role of the phoPQ operon in the pathogenesis of the fully virulent CO92 strain of Yersinia pestis and the IP32953 strain of Yersinia pseudotuberculosis.. Microb Pathog 2011; 50:314 - 21; http://dx.doi.org/10.1016/j.micpath.2011.02.005; PMID: 21320584
- Perez JC, Groisman EA. Transcription factor function and promoter architecture govern the evolution of bacterial regulons. Proc Natl Acad Sci USA 2009; 106:4319 - 24; http://dx.doi.org/10.1073/pnas.0810343106; PMID: 19251636
- Perez JC, Shin D, Zwir I, Latifi T, Hadley TJ, Groisman EA. Evolution of a bacterial regulon controlling virulence and Mg(2+) homeostasis. PLoS Genet 2009; 5:e1000428; http://dx.doi.org/10.1371/journal.pgen.1000428; PMID: 19300486
- Zhou D, Han Y, Qin L, Chen Z, Qiu J, Song Y, Li B, Wang J, Guo Z, Du Z, et al. Transcriptome analysis of the Mg2+-responsive PhoP regulator in Yersinia pestis.. FEMS Microbiol Lett 2005; 250:85 - 95; http://dx.doi.org/10.1016/j.femsle.2005.06.053; PMID: 16061330
- Zhang Y, Gao H, Wang L, Xiao X, Tan Y, Guo Z, Zhou D, Yang R. Molecular characterization of transcriptional regulation of rovA by PhoP and RovA in Yersinia pestis.. PLoS ONE 2011; 6:e25484; http://dx.doi.org/10.1371/journal.pone.0025484; PMID: 21966533
- Zhang Y, Wang L, Han Y, Yan Y, Tan Y, Zhou L, Cui Y, Du Z, Wang X, Bi Y, et al. Autoregulation of PhoP/PhoQ and positive regulation of the cyclic AMP receptor protein-cyclic AMP complex by PhoP in Yersinia pestis.. J Bacteriol 2013; 195:1022 - 30; http://dx.doi.org/10.1128/JB.01530-12; PMID: 23264579
- Heroven AK, Sest M, Pisano F, Scheb-Wetzel M, Steinmann R, Böhme K, Klein J, Münch R, Schomburg D, Dersch P. Crp induces switching of the CsrB and CsrC RNAs in Yersinia pseudotuberculosis and links nutritional status to virulence. Front Cell Infect Microbiol 2012; 2:158; PMID: 23251905
- Ellison DW, Miller VL. Regulation of virulence by members of the MarR/SlyA family. Curr Opin Microbiol 2006; 9:153 - 9; http://dx.doi.org/10.1016/j.mib.2006.02.003; PMID: 16529980
- Cathelyn JS, Ellison DW, Hinchliffe SJ, Wren BW, Miller VL. The RovA regulons of Yersinia enterocolitica and Yersinia pestis are distinct: evidence that many RovA-regulated genes were acquired more recently than the core genome. Mol Microbiol 2007; 66:189 - 205; http://dx.doi.org/10.1111/j.1365-2958.2007.05907.x; PMID: 17784909
- Cathelyn JS, Crosby SD, Lathem WW, Goldman WE, Miller VL. RovA, a global regulator of Yersinia pestis, specifically required for bubonic plague. Proc Natl Acad Sci USA 2006; 103:13514 - 9; http://dx.doi.org/10.1073/pnas.0603456103; PMID: 16938880
- Nagel G, Lahrz A, Dersch P. Environmental control of invasin expression in Yersinia pseudotuberculosis is mediated by regulation of RovA, a transcriptional activator of the SlyA/Hor family. Mol Microbiol 2001; 41:1249 - 69; http://dx.doi.org/10.1046/j.1365-2958.2001.02522.x; PMID: 11580832
- Heroven AK, Dersch P. RovM, a novel LysR-type regulator of the virulence activator gene rovA, controls cell invasion, virulence and motility of Yersinia pseudotuberculosis.. Mol Microbiol 2006; 62:1469 - 83; http://dx.doi.org/10.1111/j.1365-2958.2006.05458.x; PMID: 17074075
- Dube PH, Handley SA, Revell PA, Miller VL. The rovA mutant of Yersinia enterocolitica displays differential degrees of virulence depending on the route of infection. Infect Immun 2003; 71:3512 - 20; http://dx.doi.org/10.1128/IAI.71.6.3512-3520.2003; PMID: 12761136
- Revell PA, Miller VL. A chromosomally encoded regulator is required for expression of the Yersinia enterocolitica inv gene and for virulence. Mol Microbiol 2000; 35:677 - 85; http://dx.doi.org/10.1046/j.1365-2958.2000.01740.x; PMID: 10672189
- Heroven AK, Nagel G, Tran HJ, Parr S, Dersch P. RovA is autoregulated and antagonizes H-NS-mediated silencing of invasin and rovA expression in Yersinia pseudotuberculosis.. Mol Microbiol 2004; 53:871 - 88; http://dx.doi.org/10.1111/j.1365-2958.2004.04162.x; PMID: 15255899
- Herbst K, Bujara M, Heroven AK, Opitz W, Weichert M, Zimmermann A, Dersch P. Intrinsic thermal sensing controls proteolysis of Yersinia virulence regulator RovA. PLoS Pathog 2009; 5:e1000435; http://dx.doi.org/10.1371/journal.ppat.1000435; PMID: 19468295
- Heroven AK, Böhme K, Rohde M, Dersch P. A Csr-type regulatory system, including small non-coding RNAs, regulates the global virulence regulator RovA of Yersinia pseudotuberculosis through RovM. Mol Microbiol 2008; 68:1179 - 95; http://dx.doi.org/10.1111/j.1365-2958.2008.06218.x; PMID: 18430141
- Heroven AK, Böhme K, Dersch P. The Csr/Rsm system of Yersinia and related pathogens: a post-transcriptional strategy for managing virulence. RNA Biol 2012; 9:379 - 91; http://dx.doi.org/10.4161/rna.19333; PMID: 22336760
- Romeo T, Vakulskas CA, Babitzke P. Post-transcriptional regulation on a global scale: form and function of Csr/Rsm systems. Environ Microbiol 2012; 15:313 - 24; http://dx.doi.org/10.1111/j.1462-2920.2012.02794.x; PMID: 22672726
- Babitzke P, Romeo T. CsrB sRNA family: sequestration of RNA-binding regulatory proteins. Curr Opin Microbiol 2007; 10:156 - 63; http://dx.doi.org/10.1016/j.mib.2007.03.007; PMID: 17383221
- Timmermans J, Van Melderen L. Post-transcriptional global regulation by CsrA in bacteria. Cell Mol Life Sci 2010; 67:2897 - 908; http://dx.doi.org/10.1007/s00018-010-0381-z; PMID: 20446015
- Grabenstein JP, Marceau M, Pujol C, Simonet M, Bliska JB. The response regulator PhoP of Yersinia pseudotuberculosis is important for replication in macrophages and for virulence. Infect Immun 2004; 72:4973 - 84; http://dx.doi.org/10.1128/IAI.72.9.4973-4984.2004; PMID: 15321989
- Chavez RG, Alvarez AF, Romeo T, Georgellis D. The physiological stimulus for the BarA sensor kinase. J Bacteriol 2010; 192:2009 - 12; http://dx.doi.org/10.1128/JB.01685-09; PMID: 20118252
- Mondragón V, Franco B, Jonas K, Suzuki K, Romeo T, Melefors O, Georgellis D. pH-dependent activation of the BarA-UvrY two-component system in Escherichia coli.. J Bacteriol 2006; 188:8303 - 6; http://dx.doi.org/10.1128/JB.01052-06; PMID: 16980446
- Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 2003; 31:3406 - 15; http://dx.doi.org/10.1093/nar/gkg595; PMID: 12824337
- Bordi C, Lamy MC, Ventre I, Termine E, Hachani A, Fillet S, Roche B, Bleves S, Méjean V, Lazdunski A, et al. Regulatory RNAs and the HptB/RetS signalling pathways fine-tune Pseudomonas aeruginosa pathogenesis. Mol Microbiol 2010; 76:1427 - 43; http://dx.doi.org/10.1111/j.1365-2958.2010.07146.x; PMID: 20398205
- Brencic A, Lory S. Determination of the regulon and identification of novel mRNA targets of Pseudomonas aeruginosa RsmA. Mol Microbiol 2009; 72:612 - 32; http://dx.doi.org/10.1111/j.1365-2958.2009.06670.x; PMID: 19426209
- Brencic A, McFarland KA, McManus HR, Castang S, Mogno I, Dove SL, Lory S. The GacS/GacA signal transduction system of Pseudomonas aeruginosa acts exclusively through its control over the transcription of the RsmY and RsmZ regulatory small RNAs. Mol Microbiol 2009; 73:434 - 45; http://dx.doi.org/10.1111/j.1365-2958.2009.06782.x; PMID: 19602144
- Groisman EA, Mouslim C. Sensing by bacterial regulatory systems in host and non-host environments. Nat Rev Microbiol 2006; 4:705 - 9; http://dx.doi.org/10.1038/nrmicro1478; PMID: 16894339
- Simonet M, Richard S, Berche P. Electron microscopic evidence for in vivo extracellular localization of Yersinia pseudotuberculosis harboring the pYV plasmid. Infect Immun 1990; 58:841 - 5; PMID: 2307522
- Pujol C, Bliska JB. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis.. Infect Immun 2003; 71:5892 - 9; http://dx.doi.org/10.1128/IAI.71.10.5892-5899.2003; PMID: 14500510
- Parkhill J, Wren BW, Thomson NR, Titball RW, Holden MT, Prentice MB, Sebaihia M, James KD, Churcher C, Mungall KL, et al. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 2001; 413:523 - 7; http://dx.doi.org/10.1038/35097083; PMID: 11586360
- Song Y, Tong Z, Wang J, Wang L, Guo Z, Han Y, Zhang J, Pei D, Zhou D, Qin H, et al. Complete genome sequence of Yersinia pestis strain 91001, an isolate avirulent to humans. DNA Res 2004; 11:179 - 97; http://dx.doi.org/10.1093/dnares/11.3.179; PMID: 15368893
- Grabenstein JP, Fukuto HS, Palmer LE, Bliska JB. Characterization of phagosome trafficking and identification of PhoP-regulated genes important for survival of Yersinia pestis in macrophages. Infect Immun 2006; 74:3727 - 41; http://dx.doi.org/10.1128/IAI.00255-06; PMID: 16790745
- O’Loughlin JL, Spinner JL, Minnich SA, Kobayashi SD. Yersinia pestis two-component gene regulatory systems promote survival in human neutrophils. Infect Immun 2010; 78:773 - 82; http://dx.doi.org/10.1128/IAI.00718-09; PMID: 19933831
- Quade N, Mendonca C, Herbst K, Heroven AK, Ritter C, Heinz DW, Dersch P. Structural basis for intrinsic thermosensing by the master regulator RovA of Yersinia. J Biol Chem 2012; 287:35796 - 803; http://dx.doi.org/10.1074/jbc.M112.379156
- Miller JH. A short course in bacterial genetic: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Habor, New York, 1992
- Sambrook J. Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratories, Cold Spring Harbor, NY, 2001
- Schweer J, Kulkarni D, Kochut A, Pezoldt J, Pisano F, Pils MC, Genth H, Huehn J, Dersch P. The cytotoxic necrotizing factor of Yersinia pseudotuberculosis (CNFY) enhances inflammation and Yop delivery during infection by activation of Rho GTPases. PLoS Pathog 2013; 9:e1003746; http://dx.doi.org/10.1371/journal.ppat.1003746; PMID: 24244167
- Böhme K, Steinmann R, Kortmann J, Seekircher S, Heroven AK, Berger E, Pisano F, Thiermann T, Wolf-Watz H, Narberhaus F, et al. Concerted actions of a thermo-labile regulator and a unique intergenic RNA thermosensor control Yersinia virulence. PLoS Pathog 2012; 8:e1002518; http://dx.doi.org/10.1371/journal.ppat.1002518; PMID: 22359501
