Abstract
Mammalian mitogen-activated protein kinase (MAPK) signaling pathways respond to diverse extracellular signals and coordinate a range of cellular responses. Mixed lineage kinase 3 (MLK3) is a member of the mixed lineage kinase family of MAPK kinase kinases (MAP3Ks) that functions to regulate multiple MAPK signaling pathways. Activated forms of the Rho GTPases, Rac and Cdc42, interact with MLK3 through the Cdc42/Rac-interactive binding (CRIB) motif and promote MLK3 catalytic activity. Our recent findings demonstrate that merlin, the product of the neurofibromatosis type 2 (NF2) tumor suppressor gene, is a physiological inhibitor of MLK3. Our results suggest that merlin inhibits MLK3 activity by blocking the Cdc42-MLK3 interaction. In this commentary, the effect of merlin on Cdc42-mediated activation of MLK3 and MAPK signaling will be discussed.
Small GTPases in MAPK Signaling
The Rho and Ras subfamilies of the Ras superfamily of monomeric GTP-binding proteins act as molecular switches to regulate essential cellular processes.Citation1 The Ras proteins control cellular and developmental events such as DNA synthesis, gene expression and transformation.Citation2 The Rho subfamilies which include the Cdc42 (Cdc42Hs and G25K), Rac (1, 2 and 3 isoforms) and Rho (A, B and C isoforms), have major roles in mediating organization and assembly of the actin cytoskeleton.Citation2 Rho proteins are involved in regulating stress fiber and focal adhesion formation, Rac proteins induce actin polymerization and lamellopodia formation, and Cdc42 proteins initiate filopodia formation.Citation3 GTPases cycle between an active GTP-bound and inactive GDP-bound state and specific proteins that can affect this cycling include the guanine nucleotide exchange factors (GEFs) that catalyze the exchange of GDP for GTP, the GTPase activating proteins (GAPs) that stimulate intrinsic GTPase activity, and the guanine nucleotide dissociation inhibitors (GDIs) that inhibit GDP dissociation.Citation3 Once activated, Rho GTPases regulate actin cytoskeleton organization and other biochemical pathways including the mitogen-activated protein kinase (MAPK), nuclear factorκB (NFκB) and G1 cell cycle progression.Citation3
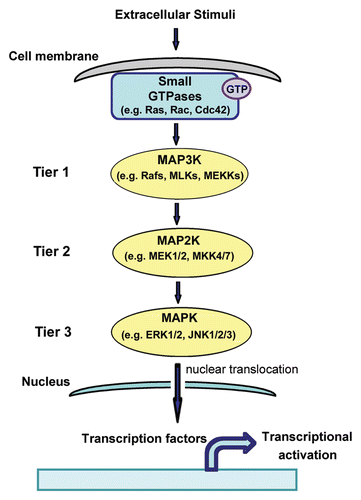
Mammalian MAPK signaling pathways function to integrate diverse extracellular stimuli and coordinate activation of cellular responses such as proliferation, survival, inflammation and metabolism.Citation4 A central feature of MAPK signaling pathways is the organization in a three-tiered protein kinase cascade where an activated MAPK kinase kinase (MAP3K) phosphorylates and activates a MAPK kinase (MAP2K), which in turn phosphorylates and activates a MAPK ().Citation4 Activated MAPKs can translocate to the nucleus where they phosphorylate transcription factors to promote activation of specific genes that regulate diverse cellular responses.Citation4 Specific Ras and Rho small GTPases can activate MAPK signaling pathways by contributing to the activation of MAP3Ks (). For instance, the direct interaction between small-GTPases (K-Ras, N-Ras and H-Ras) and the Raf MAP3Ks (Raf-1, A-Raf and B-Raf), mediated by an N-terminal Ras binding domain in the Rafs, facilitates recruitment of Rafs to the plasma membrane where a number of events, including phosphorylation by other kinases, contributes to their full enzymatic activation.Citation5 Activated Rafs phosphorylate and activate the MAP2Ks, MEK1 and 2, which in turn phosphorylate and activate the MAPKs, extracellular-stimulus regulated kinase (ERK)1 and 2.Citation6 In a similar manner, activated Rac1 or Cdc42 interact with and promote activation of MAP3Ks such as mixed lineage kinase 3 (MLK3), MEKK1 and 4.Citation7,Citation8 MLKs and MEKKs phosphorylate and activate the MAP2Ks, MKK4 and 7, which in turn phosphorylate and activate the MAPKs, c-Jun N-terminal kinase (JNK)1, 2 and 3.Citation4 Rac1 and Cdc42 also interact with other effectors such as p21-activated kinase (PAK) 1 and 2 (through the PAK binding domain) which enhances PAK1 catalytic activity.Citation9
Cdc42-mediated MLK3 Activation is Blocked by Merlin
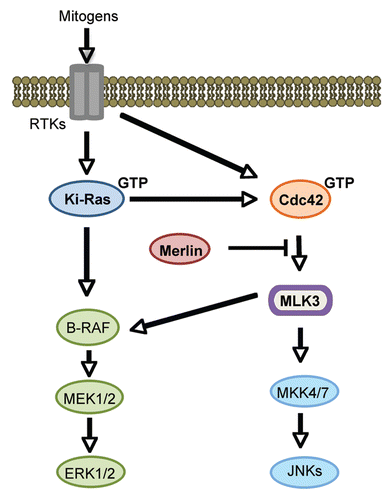
GTP-bound Cdc42 interacts with the MLK3 Cdc42/Rac interaction and binding (CRIB) domain and promotes autophosphorylation, activation and relocalization of the enzyme to the plasma membrane.Citation7,Citation10 Autoinhibition of MLK3 enzymatic activity is mediated by an interaction between the N-terminal SH3 domain and the proline residue 495, causing a ‘closed’ structure, that prevents MLK3 activation.Citation11 In a proposed model for Cdc42-mediated MLK3 activation, Cdc42 binding to the MLK3 CRIB domain blocks the association between the N-terminus of MLK3 and proline 495, which relieves MLK3 autoinhibition and promotes an open structure that facilitates autophosphorylation and activation of the enzyme.Citation10 Activated MLK3 mediates JNK activation through direct phosphorylation and activation of MKK4 and 7.Citation12,Citation13 Furthermore, a requirement for MLK3 in mitogen-dependent ERK activation has also been demonstrated.Citation14
In recent studies, we identified MLK3 as a binding partner of the tumor suppressor protein, merlin.Citation15,Citation16 Merlin is encoded by the neurofibromatosis type 2 (NF2) gene, and has homology with the Ezrin, Radixin and Moesin (ERM) group of cellular proteins.Citation17,Citation18 Merlin is an adaptor protein that links integral membrane proteins to the actin cytoskeleton and negatively regulates cell size, motility and proliferation.Citation19 Germline NF2 gene mutations predispose individuals to the development of nervous system tumors, including schwannomas, meningiomas and ependymomas.Citation20
A number of studies have demonstrated that merlin expression inhibits signaling mediated by Ras and Rac1, through inhibiting Pak and Raf-1, which could result in negative regulation of both JNK and ERK signaling.Citation21–Citation27 Merlin also negatively affects Ral GTPases by inhibiting the activity of the Ral GTPase guanine nucleotide dissociation stimulator (RalGDS).Citation28 However, very little is known about the effect of merlin expression on Cdc42 activity.
In our recent study, we demonstrate that merlin directly interacts with and potently inhibits MLK3 activity.Citation15 The C-terminal residues 340–590 of merlin directly bound to MLK3 and inhibited MLK3 kinase activity in vitro.Citation15 In addition, knockdown of merlin elevated the levels of active MLK3, ERK and JNK in ovarian tumor cells; whereas overexpression of merlin reduced the levels of active MLK3, ERK and JNK in immortalized ovarian and schwann cells.Citation15 We reasoned that merlin may prevent MLK3 activation by directly inhibiting Cdc42 activity. However, RNA interference (RNAi)—mediated merlin knockdown in HEK293 cells did not affect Cdc42 activity. Furthermore, merlin overexpression in HEK293 cells also had little effect on Cdc42 activity. Thus, in contrast to its effect on some other small GTPases, our results suggest that merlin does not directly inhibit Cdc42 activity.Citation15 Interestingly, we observed that merlin expression substantially reduced the amount of MLK3 bound to Cdc42 (wildtype) or constitutively active Cdc42 (V12) in Cdc42 co-immunoprecipitates from HEK293 cells.Citation15 These results suggest that merlin may inhibit GTP-Cdc42-mediated activation of MLK3 by blocking the Cdc42-MLK3 interaction. Interestingly, we previously observed that the MLK3-B-Raf interaction was also inhibited by merlin.Citation16 Further studies to determine the region of MLK3 that binds to merlin will be important to clearly define the mechanism by which merlin inhibits the Cdc42-MLK3 interaction. Possibly, merlin binds to a region proximal to the MLK3 CRIB domain, which could directly inhibit GTP-Cdc42 binding to MLK3.
Collectively, the results of our studies suggest that inhibition of MLK3 may be a novel mechanistic link by which merlin can suppress both Rho-GTPase and MAPK signaling. In our proposed model, the direct binding of merlin to MLK3 blocks the Cdc42-MLK3 interaction, thereby preventing MLK3-dependent activation of ERK and JNK signaling (). We postulate that merlin loss or expression of non-functional merlin in tumor cells could foster the formation of GTP-Cdc42-MLK3 complexes that could facilitate activation of JNK and ERK respectively. Further investigation will be needed to determine if elevated Cdc42-mediated activation of MLK3 drives neoplastic progression in cells that lack functional merlin.
Figures and Tables
Acknowledgements
The work was supported by National Institutes of Health Grant 1 R15 CA132006-01 and by an American Cancer Society (Ohio Division) grant (to D.N.C.).
Extra View to: Zhan Y, Modi N, Stewart AM, Hieronimus RI, Liu J, Gutmann DH, et al. Regulation of mixed lineage kinase 3 is required for neurofibromatosis-2-mediated growth suppression in human cancer. Oncogene 2011; 30:781 - 789; PMID: 20890305; http://dx.doi.org/10.1038/onc.2010.453
References
- Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J 2000; 348:241 - 255
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene 2007; 26:3291 - 3310
- Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 2005; 21:247 - 269
- Kyriakis JM, Avruch J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 2001; 81:807 - 869
- Mercer KE, Pritchard CA. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta 2003; 1653:25 - 40
- Shaul YD, Seger R. The MEK/ERK cascade: from signaling specificity to diverse functions. Biochim Biophys Acta 2007; 1773:1213 - 1226
- Du Y, Bock BC, Schachter KA, Chao M, Gallo KA. Cdc42 induces activation loop phosphorylation and membrane targeting of mixed lineage kinase 3. J Biol Chem 2005; 280:42984 - 42993
- Fanger GR, Johnson NL, Johnson GL. MEK kinases are regulated by EGF and selectively interact with Rac/Cdc42. EMBO J 1997; 16:4961 - 4972
- Lu W, Mayer BJ. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene 1999; 18:797 - 806
- Gallo KA, Johnson GL. Mixed-lineage kinase control of JNK and p38 MAPK pathways. Nat Rev Mol Cell Biol 2002; 3:663 - 672
- Zhang H, Gallo KA. Autoinhibition of mixed lineage kinase 3 through its Src homology 3 domain. J Biol Chem 2001; 276:45598 - 45603
- Tibbles LA, Ing YL, Kiefer F, Chan J, Iscove N, Woodgett JR, et al. MLK-3 activates the SAPK/JNK and p38/RK pathways via SEK1 and MKK3/6. EMBO J 1996; 15:7026 - 7035
- Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, et al. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem 1996; 271:19025 - 19028
- Chadee DN, Kyriakis JM. MLK3 is required for mitogen activation of B-Raf, ERK and cell proliferation. Nat Cell Biol 2004; 6:770 - 776
- Zhan Y, Modi N, Stewart AM, Hieronimus RI, Liu J, Gutmann DH, et al. Regulation of mixed lineage kinase 3 is required for Neurofibromatosis-2-mediated growth suppression in human cancer. Oncogene 2011; 30:781 - 789
- Chadee DN, Xu D, Hung G, Andalibi A, Lim DJ, Luo Z, et al. Mixed-lineage kinase 3 regulates B-Raf through maintenance of the B-Raf/Raf-1 complex and inhibition by the NF2 tumor suppressor protein. Proc Natl Acad Sci USA 2006; 103:4463 - 4468
- Sun CX, Robb VA, Gutmann DH. Protein 4.1 tumor suppressors: getting a FERM grip on growth regulation. J Cell Sci 2002; 115:3991 - 4000
- Ramesh V. Merlin and the ERM proteins in Schwann cells, neurons and growth cones. Nat Rev Neurosci 2004; 5:462 - 470
- Stamenkovic I, Yu Q. Merlin, a “magic” linker between extracellular cues and intracellular signaling pathways that regulate cell motility, proliferation and survival. Curr Protein Pept Sci 2010; 11:471 - 484
- Reed N, Gutmann DH. Tumorigenesis in neurofibromatosis: new insights and potential therapies. Trends Mol Med 2001; 7:157 - 162
- Kaempchen K, Mielke K, Utermark T, Langmesser S, Hanemann CO. Upregulation of the Rac1/JNK signaling pathway in primary human schwannoma cells. Hum Mol Genet 2003; 12:1211 - 1221
- Shaw RJ, Paez JG, Curto M, Yaktine A, Pruitt WM, Saotome I, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell 2001; 1:63 - 72
- Kim H, Lim JY, Kim YH, Kim H, Park SH, Lee KH, et al. Inhibition of ras-mediated activator protein 1 activity and cell growth by merlin. Mol Cells 2002; 14:108 - 114
- Tikoo A, Varga M, Ramesh V, Gusella J, Maruta H. An anti-Ras function of neurofibromatosis type 2 gene product (NF2/Merlin). J Biol Chem 1994; 269:23387 - 23390
- Lim JY, Kim H, Jeun SS, Kang SG, Lee KJ. Merlin inhibits growth hormone-regulated Raf-ERKs pathways by binding to Grb2 protein. Biochem Biophys Res Commun 2006; 340:1151 - 1157
- Kissil JL, Wilker EW, Johnson KC, Eckman MS, Yaffe MB, Jacks T. Merlin, the product of the Nf2 tumor suppressor gene, is an inhibitor of the p21-activated kinase, Pak1. Mol Cell 2003; 12:841 - 849
- Morrison H, Sperka T, Manent J, Giovannini M, Ponta H, Herrlich P. Merlin/neurofibromatosis type 2 suppresses growth by inhibiting the activation of Ras and Rac. Cancer Res 2007; 67:520 - 527
- Ryu CH, Kim SW, Lee KH, Lee JY, Kim H, Lee WK, et al. The merlin tumor suppressor interacts with Ral guanine nucleotide dissociation stimulator and inhibits its activity. Oncogene 2005; 24:5355 - 5364