Abstract
Despite being obligately associated with warm-blooded animals, Candida albicans expresses a bona fide heat shock response that is regulated by the evolutionarily conserved, essential heat shock transcription factor Hsf1. Hsf1 is thought to play a fundamental role in thermal homeostasis, adjusting the levels of essential chaperones to changes in growth temperature, for example in febrile patients. Hsf1 also regulates the expression of Hsp90, which controls the yeast-hypha transition in C. albicans, and we argue, might also control morphogenesis in other fungal pathogens of humans.
Numerous observations link the heat shock response with the pathogenicity of the major fungal pathogen of humans, Candida albicans. Firstly, yeast-hypha morphogenesis is considered to be a virulence attribute and mild heat shock is integral to most experimental conditions that are used to stimulate hyphal development in vitro.Citation1–Citation3 Secondly, the induction of heat shock protein expression is associated with morphogenesis,Citation3,Citation4 which is not surprising given the concomitant temperature upshift used to promote hyphal development. Thirdly, heat shock proteins are found at the C. albicans cell surface and these proteins are immunogenic during infections.Citation5–Citation9 Fourthly, immunization with Hsp70 appears to sensitise experimental animals to systemic Candida infection, reducing their survival time apparently by hyperactivating their immune systems.Citation8 Fifthly, in contrast to the situation with Hsp70, autoantibodies against Hsp90 are immunoprotective, providing a degree of protection against subsequent intravenous infection with C. albicans,Citation10 and this has underpinned the development of a new antifungal therapeutic, Mycograb.Citation11 Therefore heat shock proteins have long been of interest to the medical mycology community.
Studies of the regulation of other stress responses in C. albicans have indicated that key stress regulators have been evolutionarily conserved in this pathogen compared with its benign cousin, the model yeast Saccharomyces cerevisiae. However, in some cases these stress regulators have been rewired such that their cellular roles have changed. For example, the Hog1 stress activated protein kinase primarily regulates osmotic stress responses in S. cerevisiae, whereas it also contributes to oxidative, heavy metal and cell wall stresses, and responses to antifungals and quorum sensing molecules in C. albicans.Citation12–Citation14 Furthermore, the transcription factors Msn2 and Msn4 play key roles in the core stress response in S. cerevisiae, unlike their homologues in C. albicans.Citation15,Citation16 In contrast the AP-1-like transcription factors Cap1 and Yap1 both play central roles in the oxidative stress responses in both C. albicans and S. cerevisiae, respectively.Citation17,Citation18
Given this background, we became intrigued by the heat shock response in C. albicans. Rather naively, we wondered whether a bona fide heat shock response is evolutionarily conserved in this fungal pathogen, which is apparently obligately associated with warm-blooded animals.Citation1 Therefore, we tested this using a combination of reductionist and genome-wide approaches, confirming that C. albicans does express a bona fide heat shock response, activating HSE-(heat shock element)-containing genes in response to an acute thermal upshift via the evolutionarily conserved heat shock transcription factor, Hsf1.Citation19
Why has a heat shock response been conserved in C. albicans? The answer lies partly in the observation that Hsf1 is essential for viability in C. albicans, as in other yeasts.Citation19 This essentiality appears to be due to the fact that Hsf1 is required for the basal expression of key chaperones that are required for protein folding, even in the absence of heat shock.Citation19 However, this explanation seemed unsatisfactory at the time because, given the extent of transcriptional rewiring in C. albicans,Citation15,Citation16,Citation20–Citation22 couldn't alternative regulators have evolved to drive the basal expression of such vital chaperones? Therefore, we reasoned that the role of Hsf1 might have diverged in C. albicans such that it contributes to other stress responses in this pathogen. We tested this only to find that the Hsf-dependent HSE-reporter is not activated in C. albicans by the other stresses we tested.Citation19
At this point we reconsidered the possible roles of the heat shock response in the wild. Molecular biologists regularly apply acute insults to provoke dramatic molecular responses that are easy to assay experimentally. However, in reality the cellular systems we study may have evolved to maintain homeostasis in the face of more modest environmental changes. A good example of this is the osmotic stress response in S. cerevisiae, which appears perfectly adapted to the maintenance of homeostasis when faced with repeated osmotic challenges.Citation23 By analogy, the heat shock response may have evolved in C. albicans to maintain homeostasis in response to less acute thermal challenges than experimentalists tend to apply in vitro. This pathogen, which probably evolved as a commensal organism, is likely to be exposed to modest thermal challenges such as, for example, those imposed by a febrile patient, or by the impact of ambient temperature upon the skin. We tested this idea by measuring the degree of activation of the Hsf1-HSE regulon at different growth temperatures, finding that the activity of this regulon increased as the growth temperature increased above 30°C.Citation19 In light of these observations, we suggest that the primary role of Hsf1 in the wild is to act as a thermostat that tunes the levels of essential chaperones to growth temperature. In this case “heat shock” is probably a misnomer for this transcription factor, and the term “thermosensor” might be more appropriate.
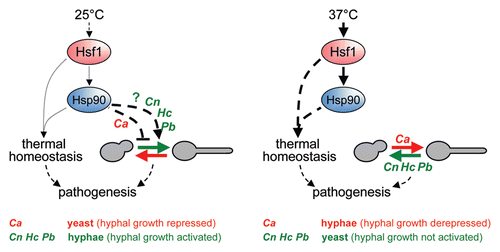
Hsp90 is an essential chaperone that is regulated by Hsf1 in C. albicans (). Recently, Leah Cowen's group elegantly demonstrated that Hsp90 plays a central role in the thermoregulation of the yeast-hypha transition. They showed that the heat shock protein Hsp90 negatively regulates hyphal development by repressing Ras-protein kinase A (PKA) signalling.Citation24 The downregulation of Hsp90 activity, either by genetic or pharmacological means, derepressed filamentation in a manner that was dependent upon Ras1, adenylyl cyclase and PKA. Hsp90 is known to interact physically with regulators (its clients) to modulate their activity,Citation25 and this presumably underpins the ability of this chaperone to act as an “evolutionary capacitor” that affects the emergence of drug resistance in C. albicans.Citation26,Citation27 (Indeed this fundamental property of Hsp90 appears to have influenced the evolution of organisms throughout the eukaryotic kingdomCitation28). Therefore, the impact of Hsp90 upon morphogenesis is thought to be mediated via an interaction with a client on the Ras-PKA pathway,Citation29 and this mechanism is likely to be regulated in turn by Hsf1 (). Indeed, chaperonins such as the CCT complex have previously been shown to modulate Ras-PKA signalling in C. albicans,Citation30 suggesting that several classes of chaperone regulate this pathway. The conundrum is how Hsp90 can inhibit hyphal development when Hsp90 expression levels increase during hyphal development.Citation4 The answer probably lies in the sequestering of Hsp90 for protein refolding during the thermal upshift. This sequestration might release the morphogenetic regulator from Hsp90, thereby allowing this client to activate hyphal development.
Temperature also regulates morphogenetic transitions in other pathogenic fungi. Unlike C. albicans, Histoplasma capsulatum, Cryptococcus neoformans and Paracoccidiodes brasiliensis are saprobes, occupying environmental niches outside their mammalian host. These fungi all exist in filamentous forms in the environment, but grow in the yeast form in the host, and these morphogenetic transitions can be induced by thermal shifts in vitro. In H. capsulatum, C. neoformans and P. brasiliensis, Hsp90 expression levels have been shown to increase in response to the elevated temperatures that induce their yeast growth form.Citation31–Citation33 Hence the Hsf1-Hsp90 module might play a role in the regulation of morphogenesis in these fungi (). However, at least in P. brasiliensis, the increase in Hsp90 expression is transient, inferring that additional factors must play a role in the maintenance of the yeast growth form. Ras-PKA signalling would be an obvious candidate. Also, while elevated temperatures induce hyphal development in C. albicans, they promote yeast growth in these other pathogens. Therefore, Hsp90 presumably exerts opposite effects upon hyphal development in these species (). For example, the interaction of Hsp90 with a key regulator might inhibit this client in one species, but activate this client in another.
To summarise, while heat shock proteins have been of interest to the medical mycology community for several decades, recent work has now begun to shed light on the regulation of this response in C. albicans. It is now becoming clear that heat shock regulation not only impacts upon thermal homeostasis in C. albicans, but also upon morphogenesis (and hence fungal pathogenesis) and drug resistance (and hence antifungal therapy). We argue that these observations may be of relevance to other clinically relevant fungal pathogens.
Figures and Tables
Figure 1 Speculative model linking the regulation of Hsp90 by Hsf1 to morphogenesis in fungal pathogens. Hsf1 activates Hsp90 in response to temperature upshifts and promotes thermal homeostasis in C. albicans via Hsp90 and other factors.Citation19 Hsp90 negatively regulates hyphal development in C. albicans (Ca).Citation24 Temperature upshifts also induce HSP90 expression in C. neoformans (Cn), H. capsulatum (Hc) and P. brasiliensis (Pb)Citation31–Citation33 and promote yeast growth rather than hyphal development in these fungi. This raises the possibility that Hsp90 might also regulate morphogenesis in these pathogenic fungi (see text). According to this model, at 25°C free Hsp90 would inhibit hyphal development in C. albicans (such that it grows in the yeast form), but promote hyphal growth in the other pathogens. Following a temperature shift to 37°C, Hsp90 would become sequestered for protein folding thereby mediating thermal adaptation. This sequestration would lead to the dissociation of Hsp90 from its as yet unidentified client on the Ras-PKA pathway, thereby derepressing hyphal development in C. albicans. In contrast, in the other pathogens this would remove hyphal activation leading to their growth in the yeast form. It should be noted that while the yeast forms of C. neoformans, H. capsulatum and P. brasiliensis are pathogenic, both the yeast and hyphal forms of C. albicans contribute to pathogenesis.Citation34
Acknowledgements
A.B. is supported by the UK Biotechnology and Biological Research Council (BB/F00513X/1; BB/D009308/1; BB/F000111/1), the Wellcome Trust (080088) and the European Commission (PITN-GA-2008-214004; ERC-2009-AdG20090506). M.L. was supported by a Carnegie/Caledonian Scholarship from The Carnegie Trust. S.N. was supported by the BBSRC (BB/D009308/1).
Addendum to:
References
- Odds FC. Candida and candidosis: a review and bibliography 1988; 2nd ed. London Bailliere Tindall
- Buffo J, Herman MA, Soll DR. A characterization of pH regulated dimorphism in Candida albicans. Mycopathol 1984; 86:21 - 30
- Dabrowa N, Howard DH. Heat shock and heat stroke proteins observed during germination of the blastoconidia of Candida albicans. Infect Immun 1984; 44:537 - 539
- Swoboda RK, Bertram G, Budge S, Gooday GW, Gow NAR, Brown AJP. Structure and regulation of the Hsp90 gene from the pathogenic fungus Candida albicans. Infect Immun 1995; 63:4506 - 4514
- Matthews RC, Burnie JP, Tabaqchali S. Isolation of immunodominant antigens from the sera of patients with systemic candidiasis and characterisation of the serological response to Candida albicans. J Clin Microbiol 1987; 25:230 - 237
- Swoboda RK, Miyasaki S, Greenspan D, Greenspan JS. Heat-inducible ATP-binding proteins of Candida albicans are recognized by sera of infected patients. J Gen Microbiol 1993; 139:2995 - 3003
- Lopez-Ribot JL, Alloush HM, Masten BJ, Chaffin WL. Evidence for presence in the cell wall of Candida albicans of a protein related to the hsp70 family. Infect Immun 1996; 64:3333 - 3340
- Bromuro C, la Valle R, Sandini S, Urbani F, Ausiello CM, Morelli L, et al. A 70-kilodalton recombinant heat shock protein of Candida albicans is highly immunogenic and enhances systemic murine candidiasis. Infect Immun 1998; 66:2154 - 2162
- Pitarch A, Sanchez M, Nombela C, Gil C. Sequential fractionation and two-dimensional gel analysis unravels the complexity of the dimorphic fungus Candida albicans cell wall proteome. Mol Cell Proteom 2002; 1:967 - 982
- Matthews RC, Burnie JP, Howat D, Rowland T, Walton F. Autoantibody to Hsp90 can mediate protection against systemic candidosis. Immunol 1991; 74:20 - 24
- Matthews RC, Rigg G, Hodgetts S, Carter T, Chapman C, Gregory C, et al. Preclinical assessment of the efficacy of Mycograb, a human recombinant antibody against fungal Hsp90. Antimicrob Agents Chemother 2003; 47:2208 - 2216
- Alonso-Monge R, Navarro-Garcia F, Roman E, Negredo AI, Eisman B, Nombela C, et al. The Hog1 Mitogen-Activated Protein Kinase Is Essential in the Oxidative Stress Response and Chlamydospore Formation in Candida albicans. Eukaryot Cell 2003; 2:351 - 361
- Smith DA, Nicholls S, Morgan BA, Brown AJP, Quinn J. A conserved stress-activated protein kinase regulates a core stress response in the human pathogen Candida albicans. Mol Biol Cell 2004; 15:4179 - 4190
- Enjalbert B, Smith DA, Cornell MJ, Alam I, Nicholls S, Brown AJP, et al. Role of the Hog1 stress-activated protein kinase in the global transcriptional response to stress in the fungal pathogen Candida albicans. Mol Biol Cell 2006; 17:1018 - 1032
- Nicholls S, Straffon M, Enjalbert B, Nantel A, Macaskill S, Whiteway M, et al. Msn2/4-like transcription factors play no obvious roles in the stress responses of the fungal pathogen, Candida albicans. Eukaryotic Cell 2004; 3:1111 - 1123
- Ramsdale M, Selway L, Stead D, Walker J, Yin Z, Nicholls SM, et al. The novel gene MNL1 regulates weak acid induced stress responses of the fungal pathogen Candida albicans. Molec Biol Cell 2008; 19:4393 - 4403
- Alarco AM, Raymond M. The bZip transcription factor Cap1p is involved in multidrug resistance and oxidative stress response in Candida albicans. J Bacteriol 1999; 181:700 - 708
- Kuge S, Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J 1994; 13:655 - 664
- Nicholls S, Leach MD, Priest C, Brown AJP. Role of the heat shock transcription factor, Hsf1, in a major fungal pathogen that is obligately associated with warm-blooded animals. Mol Microbiol 2009; 74:844 - 861
- Ihmels J, Bergmann S, Gerami-Nejad M, Yanai I, McClellan M, Berman J, et al. Rewiring of the yeast transcriptional network through the evolution of motif usage. Science 2005; 309:938 - 940
- Hogues H, Lavoie H, Sellam A, Mangos M, Roemer T, Purisima E, et al. Transcription factor substitution during the evolution of fungal ribosome regulation. Mol Cell 2008; 29:552 - 562
- Askew C, Sellam A, Epp E, Hogues H, Mullick A, Nantel A, et al. Transcriptional regulation of carbohydrate metabolism in the human pathogen Candida albicans. PLoS Pathog 2009; 5:1000612
- Klipp E, Nordlander B, Kruger R, Gennemark P, Hohmann S. Integrative model of the response of yeast to osmotic shock. Nature Biotech 2005; 23:975 - 982
- Shapiro R, Uppuluri P, Zaas AK, Collins C, Senn H, Perfect JR, et al. Hsp90 orchestrates temperaturedependent Candida albicans morphogenesis via Ras1-PKA signalling. Current Biol 2009; 19:1 - 9
- Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog 2009; 5:1000532
- Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science 2005; 309:2185 - 2189
- Cowen LE. Hsp90 orchestrates stress response signaling governing fungal drug resistance. PLoS Pathogens 2009; 5:1000471
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature 1998; 396:336 - 342
- Shapiro R, Cowen LE. Coupling temperature sensing and development: Hsp90 regulates morphogenetic signaling in Candida albicans. Virulence 2010; 1:45 - 48
- Rademacher F, Kehren V, Stoldt VR, Ernst JF. A Candida albicans chaperonin subunit (CaCct8p) as a suppressor of morphogenesis and Ras phenotypes in C. albicans and Saccharomyces cerevisiae. Microbiol 1998; 144:2951 - 2960
- Minchiotti G, Gargano S, Maresca B. The intron-containing hsp82 gene of the dimorphic pathogenic fungus Histoplasma capsulatum is properly spliced in severe heat shock conditions. Mol Cell Biol 1991; 11:5624 - 5630
- Steen BR, Lian T, Zuyderduyn S, MacDonald WK, Marra M, Jones SJ, et al. Temperature-regulated transcription in the pathogenic fungus Cryptococcus neoformans. Genome Res 2002; 12:1386 - 1400
- Nicola AM, Andrade RV, Dantas AS, Andrade PA, Arraes FBM, Fernandes L, et al. The stress responsive and morphologically regulated hsp90 gene from Paracoccidioides brasiliensis is essential to cell viability. BMC Microbiol 2008; 8:158
- Gow NAR, Brown AJP, Odds FC. Fungal morphogenesis and host invasion. Current Opin Microbiol 2002; 5:366 - 371