Abstract
Fungal meningitis is a serious disease caused by a fungal infection of the central nervous system (CNS) mostly in individuals with immune system deficiencies. Fungal meningitis is often fatal without proper treatment, and the mortality rate remains unacceptably high even with antifungal drug interventions. Currently, cryptococcal meningitis is the most common fungal meningitis in HIV-1/AIDS, and its disease mechanism has been extensively studied. The key steps for fungi to infect brain and cause meningitis after establishment of local infection are the dissemination of fungal cells to the bloodstream and invasion through the blood brain barrier to reach the CNS. In this review, we use cryptococcal CNS infection as an example to describe the current molecular understanding of fungal meningitis, including the establishment of the infection, dissemination, and brain invasion. Host and microbial factors that contribute to these infection steps are also discussed.
General Causes of Meningitis
Meningitis refers to inflammation of the meninges (membranes that cover the brain and the spinal cord), which is commonly caused by infection with pathogens, including bacteria, fungi, viruses or parasites. Despite advances in antimicrobial and antiviral therapy, meningitis still results in significant morbidity and mortality.Citation1 The most common symptoms of meningitis are headache, neck stiffness and pain, with associated fever, vomiting, seizures, confusion or altered consciousness and an inability to tolerate light or loud noises.Citation2
The key step for a pathogen to infect brain and cause meningitis is to cross the blood brain barrier (BBB), an interface that separates the peripheral circulation and the central nervous system (CNS). The BBB occurs along all capillaries in the CNS and consists of tight junctions around the capillaries to regulate the passage of blood-borne substances into the brain and maintain the homeostasis of the neural microenvironment that is crucial for normal neuronal function (). This specialized system restricts microbe and large molecules in blood from entry into the brain, while allowing the diffusion of small hydrophobic molecules such as O2, hormones and CO2.Citation3,Citation4
Figure 1. (A) The illustration of the blood-brain barrier (BBB). The BBB is a multi-cellular structure at the interface of circulation and the central nervous system. It is composed of brain microvascular endothelial cells, astrocytes, pericytes and neurons. The main function of the BBB is to maintain the neural microenvironment by regulating the changes of the levels of molecules in the blood, and protect the brain by blocking the entry of toxins and microorganisms that are circulating in the blood. (B) Pathogens can cross the BBB transcellularly, paracellularly and/or in infected phagocytes (the Trojan horse mechanism). In the transcellular traversal model (a), pathogens across the barrier by direct endocytosis of brain microvascular endothelial cells without disruption of intercellular tight junction. In the Paracellular traversal model (b), pathogens penetrate between barrier cells through loosen tight junction, and may or may not lead to tight-junction disruption. The “Trojan horse” mechanism (c) involves phagocytic microbial penetration of the barrier cells using transmigration within infected phagocytes. Pathogen cells are released from macrophages after penetration.
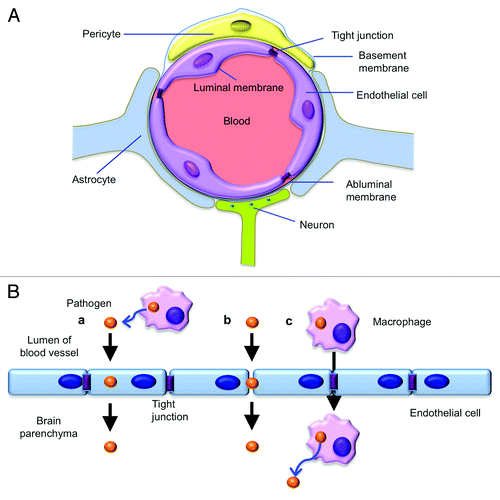
Only limited numbers of pathogens are capable of invading the brain to cause meningitis. To successfully infect the brain, pathogens have evolved various strategies to penetrate the BBB. Pathogens may cross the BBB transcellularly, paracellularly and/or by the so-called “Trojan horse” mechanism.Citation5 Transcellular traversal refers to microbial penetration through human brain microvascular endothelial cells (HBMECs) without disrupting intercellular tight junctions. Paracellular traversal is defined as microbial penetration between barrier cells with and/or without disruption of tight junctions. This mechanism involves loosening of the tight junction or disrupting of its supporting components, such as glial cells and basement membrane.Citation6 The “Trojan horse” mechanism involves microbial penetration of the barrier cells using transmigration within infected phagocytesCitation4 (). All three mechanisms have been reported in bacterial pathogens to gain entry, while the “Trojan horse” mechanism is common in virus infection, such as HIV-1 and West Nile virus.Citation4
A number of fungi have been shown to cause meningitis in humans, including yeast pathogens (Cryptococcus neoformans and Candida albicans), dimorphic fungi (Histoplasma capsulatum, Coccidioides immitis, Paracoccidioides brasiliensis and Blastomyces dermatitidis),Citation7 filamentous fungi (Aspergillus species and Zygomycetes) and several dematiaceous molds (Bipolaris spicifera, Exophiala jeanselmei, Cladophialophora bantiana, Ochroconis gallopavum and Ramichloridium mackenziei)Citation8-Citation10 (). Cryptococcus neoformans is the causative agent for the most common fungal meningitis, especially in areas where HIV-1 is prevalent. Cryptococcal meningitis is uniformly fatal without proper treatment.Citation11,Citation12 It is also the most extensively studied type of fungal meningitis. In this review, we use cryptococcal meningitis as a model to describe the current understanding of fungal meningitis.
Table 1. Causative agents of fungal meningitis
Development of Cryptococcosis
Significance of cryptococcal meningitis
C. neoformans causes the most common fungal infection of the central nervous system (CNS) in HIV-1/AIDS populations with high mortality and morbidity. CNS cryptococcosis may present as encephalitis, meningitis or cerebral-space-occupying lesions. It is reported that cryptococcal meningitis occurs in 8% of patients with HIV/AIDS in the US and as much as 40% of these patients in other part of the world.Citation13 A recent epidemiologic analysis projected that there are around one million cases of cryptococcal meningitis in AIDS patients each year that are responsible for over 600,000 annual deaths.Citation12
Environmental niches
There are two principal Cryptococcus species that often cause human and animal infections, C. neoformans (serotype A and D) and its sibling species C. gattii (serotype B and C). Cryptococcus exists ubiquitously in the environment with worldwide distribution. It was first isolated from peach juice samples in 1894.Citation14 There are several major environmental niches where Cryptococcus cells can be most frequently isolated. C. neoformans is commonly associated with soil and bird droppings and has a global distribution, whereas its sibling species C. gattii is traditionally a tropical and subtropical organism, associating with several tree species, including Eucalyptus spp.Citation15-Citation18
The detailed mechanism as to why Cryptococcus prefers these particular niches remains unclear. Our recent studies showed that inositol from plants plays an important role in stimulating sexual reproduction in Cryptococcus species, suggesting that Cryptococcus can utilize certain compounds from niches for its development, which may have broad implication of the host-pathogen co-evolution.Citation19 The observation that the sexual reproduction of C. neoformans occurs in media made of pigeon guano also suggests the benefit of environmental niches for the development of this microbe.Citation20 The recent outbreak of C. gattii infection in immunocompetent individuals on Vancouver Island, Canada and its expansion in Canada and the Pacific Northwest suggests an evolution of host range, geographic location, and virulence of this pathogen. This further underlines the complexity of its epidemiology and disease mechanism.Citation21-Citation25
Pulmonary infection
Cryptococcus spores, produced as a result of sexual reproduction, or desiccated yeasts are believed to be the initial infectious particles in nature, which has been supported by in vivo studies using animal models.Citation26-Citation28 Spores inhaled by human or animal hosts will lodge into lung alveoli. Cryptococcus can colonize the host respiratory tract without producing significant symptomatic disease, thus initial infection can be in a dormant or latent form. When host immunity is compromised, the dormant form may reactivate and disseminate hematogenously to cause systemic infection.Citation29 Cryptococcal exposure is prevalent, which was evident by a survey that indicates almost all adults in New York have antibody reactive to C. neoformans, an indication of exposure to this organism.Citation30
For the host, containment of fungus in the lung is accomplished with a combination of cell-mediated immunity, innate immunity, as well as antibody responses.Citation31 Macrophages are the first line of host defense and complement-mediated phagocytosis is likely to be the primary initial defense against cryptococcal infection.Citation32,Citation33 Other host factors important for defense against infection include CD4+ and CD8+ T cells, as well as cytokines such as tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-18.Citation34,Citation35 Antibodies are part of the immune response to cryptococcal infection, as animal studies have shown that cryptococcal infection treated with antibodies against the capsule component glucuronoxylomannan (GXM) can reduce the fungal burden and enhance animal survival.Citation36 If the host immune system fails to contain the fungus, Cryptococcus can infect and spread to other organs to cause infections involving almost any part of the body, including the skin, eyes, myocardium, bones, joints, lungs, prostate gland, or urinary tract, as well as the central nervous system (CNS).Citation33
Dissemination
Dissemination occurs when the host defense mechanism fails, i.e., when phagocytic cells fail to kill the yeast, instead serving as a niche for fungal replication. Fungal cells can be disseminated by moving through macrophages or other mechanisms to reach the blood circulation. Yeast cells can replicate inside macrophages to form cryptococcal phagosomes, which then lead to the burst of host macrophages to release fungal cells. Cryptococcal cells can also exit macrophages by extrusion without lysis, allowing both the host cell and pathogen to survive.Citation37-Citation39 A recent report showed that a WASP-Arp2/3 complex-mediated actin polymerization mechanism is involved in temporarily inhibiting expulsion of yeast cells from infected phagocytes.Citation40 Excited yeast cells can then be immediately internalized by another macrophage without encountering the extracellular environment to avoid the host defense system.Citation37-Citation39
Cryptococcal cells are rapidly cleared from the bloodstream by the host defense system. Studies using a murine model showed that only 1.7% to 2% of yeast cells deposited in the bloodstream through tail vein injection survived after 30 min.Citation41 One potential mechanism for yeast cells to survive and disseminate is to maintain and replicate inside macrophages, as macrophages can be utilized as a niche for replication if they fail to kill yeast cells.Citation37,Citation39 Yeast cells reach the BBB through the blood circulation and cross it to reach the meninges, where rapid replication occurs to cause inflammation and subsequent meningoencephalitis. Alternatively, macrophages containing yeast cells may be able to directly cross the BBB to deliver fungal cells inside the brain.
Both cryptococcal pneumonia and cryptococcal meningitis are serious disease manifestations and are potentially fatal. There is no clear evidence of human-to-human transmission, thus human and animals are likely the terminal host, and cells can only be recycled in nature from demise hosts.
Pathogen factors involved in the cryptococcal pulmonary infection and dissemination
There are several well-characterized virulence factors that contribute to the success of a fungal infection. Tolerance to mammalian body temperature 37°C is a precondition for the establishment of infection. The thermal tolerance of C. neoformans is linked to cell integrity and is controlled by multiple primary signaling pathways, including the calcineurin pathwayCitation42 and the protein kinase C1 (PKC1)-activated MAP kinase (Mpk1) pathway.Citation43,Citation44 Cryptococcal cells are covered by a large polysaccharide capsule, which is induced by CO2,Citation45,Citation46 phospholipidsCitation47 and low iron conditions.Citation48 Capsule formation protects cells from phagocytosis.Citation33,Citation49 Its capsular polysaccharide glucuronoxylomannan (GXM) component interferes with T-cell function, disrupting cell-mediated immunity.Citation50-Citation53 The polysaccharide capsule is also an important microbial factor affecting dissemination. Following intravenous injection in a murine model, around 0.2% CFU of an encapsulated strain was recovered in the circulating blood after 24 h of inoculation, while no yeast cells of an acapsular strain were detected after 2 h following the infection, indicating the importance of capsule in dissemination.Citation41 This finding is consistent with the role of GXM in inhibiting T-cell activation and proliferation, thus preventing cryptococcal cells from being cleared in the blood.Citation50,Citation54 In addition, Cryptococcus contains a thick cell wall with the deposition of phenolic melanin, which has been proposed to protect cells from oxidation.Citation55,Citation56 Laccase, a key enzyme required for melanin biosynthesis, is also important for fungal virulence.Citation57,Citation58 Mutant strains lacking laccase production show virulence attenuation due to inability to escape from the lungs, without affecting growth in either the blood or the brain, indicating that laccase plays a role in the dissemination of Cryptococcus into the bloodstream.Citation58 Both capsule formation and melanin production are controlled by the Gpa1 G protein signaling pathway via regulation of cellular cAMP levels.Citation59,Citation60
Enzymes involved in inositol metabolism pathways have been found to be important for pathogenicity in C. neoformans.Citation61,Citation62 Inositol sphingolipid biosynthesis and breakdown are required for Cryptococcus pathogenicity by regulating intracellular and extracellular fungal growth.Citation61,Citation63-Citation65 The inositol phosphoryl ceramide synthase 1 (Ipc1) and its downstream protein App1 (antiphagocytic protein 1) were both found to be important for regulating Cryptococcus phagocytosis and potential dissemination.Citation61,Citation66 Meanwhile, cryptococcal inositol phosphosphingolipid phospholipase C1 (Isc1), an enzyme that breaks down inositol sphingolipids, has been shown to be important for the survival of C. neoformans in activated macrophages.Citation67 The isc1Δ mutant strain produces much less fungal burden in the lung when compared with the wild type strain in a murine model, and fails to disseminate due to its inability to survive after phagocytosis by activated macrophages.
The secreted enzyme phospholipase B1 (Plb1) is also important for the dissemination of Cryptococcus into the bloodstream. Plb1 is a multifunctional enzyme containing activities of phospholipase B, lysophospholipase and lysophospholipase transacylase.Citation68,Citation69 Compared with wild-type, mice infected by plb1Δ mutant strains show a reduced fungal burden in lung interstitial macrophages and extrapulmonary sites, including brain, suggesting Plb1 is required for both the initiation of infection and dissemination.Citation70
Different fungal mating types have also been demonstrated to have different outcomes for disease progression. When animals are co-infected with mixtures of α and a mating type cells, α cells preferentially disseminate to the CNS.Citation71 Recent studies demonstrate that the pheromone sensing during co-infection in vivo leads to the production of much enlarged a cells (giant cells/titan cells) that prevent them from effectively undergoing phagocytosis.Citation71,Citation72 The size of titan cells could be as large as 100 uM in diameter, 10x larger than typical cryptococcal cells. Because survival in macrophages is important for fungal cell trafficking and dissemination, such morphological transition causes a defect in a cell dissemination from lung to the bloodstream.Citation72,Citation73 G protein-coupled receptors (GPCRs) Ste3a and Gpr5 have been shown to regulate the gigantism of cryptococcal cells via the activation of G protein Gpa1 and its downstream signaling pathway (Gpa1-Pka1-Rim101 signaling cascade).Citation74 Meanwhile, phospholipids have been reported to stimulate the giant cell formation.Citation47 It would be interesting to investigate whether certain GPCRs, such as Ste5, can sense phospholipid as a ligand.
Mechanism of Brain Infection
Penetration of the BBB is the key step for pathogens infecting brain to cause meningitis. Fungal cells are much larger in size compared with viruses or bacteria that often cause meningitis. How fungal cells cross the BBB to invade brain is a fundamental question for studying fungal meningitis. Although this key issue is not completely understood in cryptococcosis, recent research advances have suggested that multiple mechanisms likely exist. Both a direct transcellular transmigration mechanism and the “Trojan horse” model have been reported in Cryptococcus.Citation41,Citation75-Citation77
Transcellular model
The transcellular model was supported by results from experimental mouse models of cryptococcal meningitis following intravenous inoculation, as well as cases of human cryptococcal meningitis.Citation41,Citation76,Citation78 C. neoformans invasion into the brain following fungemia was reported to occur via cell entry through cerebral capillaries, not the choroid plexus.Citation79 It can enter and traverse human brain microvascular endothelial cells (HBMECs) without any obvious change in HBMEC integrity. Observations with transmission and scanning electron microscopy have revealed that C. neoformans induces the reorganization of host cell cytoskeletal structures and the formation of microvilli-like protrusions to initiate its entry into HBMECs.Citation41,Citation80 C. neoformans is found intracellularly in membrane-bound vacuoles and no free cryptococcal cells are found in the HBMEC cytoplasm. Once internalized, C. neoformans causes minimal endothelial cell damage and exits from the surface of the cell.Citation41,Citation81 These findings indicate that C. neoformans can use a transcellular mechanism to enter HBMECs that involves host cell actin cytoskeleton rearrangements. Further studies revealed that the invasion is mediated through the lipid rafts-endocytic pathway that involves the molecule ganglioside GM1, cell surface glycoprotein CD44 protein, cytoskeleton and intracellular kinase-DYRK3 (dual specificity tyrosine-phosphorylation-regulated kinase 3).Citation82,Citation83 Hyaluronic acid was identified as the fungal ligand that directly interacts with CD44 from lipid rafts during Cryptococcus adhesion and invasion of HBMEC. Also, the host cell protein kinase C α isoform has been reported to be important for the BBB crossing by regulating actin filament activity.Citation84 HBMEC treated with either PKC inhibitors or F-filament-disrupting agents inhibits fungal invasion into the endothelial monolayer.
A recent study using intravital microscopy to generate real-time imaging of cryptococcal transmigration in mouse brain elegantly show that both yeast cells and polystyrene microsphere with similar size stop moving suddenly once reach mouse brain capillaries of similar or smaller diameter than the particle, and then only viable cells can cross the capillary wall. These results indicate that cryptococcal cells are mechanically trapped in the brain capillary during dissemination but actively transmigrate to the brain parenchyma. This observation is in agreement with the transcellular model.Citation75
It has been suggested that a potential paracellular transmigration mechanism might exist in C. neoformans. A major tight junction maker protein, occludin, was found rapidly degraded in connection to the actin cytoskeletal rearrangement in endothelial cells during the Cryptococcus-HBMEC interaction, an indication that Cryptococcus might have a profound impact on the integrity of HBMEC tight junction. A loosen or disrupted tight junction might allow yeast cells to pass.Citation80 However, a direct evidence for such a mechanism is still lacking.
“Trojan horse” model
The importance of macrophages in the cryptococcal infectious cycle has been well-documented.Citation85,Citation86 Cryptococcus is a facultative intracellular pathogen that can survive in macrophages as a niche for replication and for avoiding the hostile host environment.Citation87 Yeast cells can escape alive from phagocytic cells by an active mechanism of phagosomal extrusion and then invasion of other phagocytes.Citation37-Citation39 The extrapulmonary dissemination is macrophage associated, suggesting a tight association between yeast cells and macrophages during infection.Citation67,Citation88 Cryptococcal cells were also observed to be closely associated with phagocytic cells in the meningeal vasculature,Citation76 which supports the hypothesis of the “Trojan horse” model. The direct evidence for a “Trojan horse” model came from a recent report where mice were infected with macrophages containing ingested cryptococcal cells.Citation77 In this report, bone marrow-derived monocytes (BMDM) infected with Cryptococcus were used for mouse intravenous infection. Compared with mice infected by free yeast cells, a 4-fold higher brain CFU was observed for the BMDM yeast. Late phagocyte depletion obtained by clodronate injection reduced disease severity and lowered the fungal burden by 40% in all organs studied. These results provide evidence for “Trojan horse” crossing of the BBB by C. neoformans and overall for a role of phagocytes in fungal dissemination.Citation77
Virulence factors important for brain infection
Studies have shown that various cryptococcal virulence factors contribute to extrapulmonary dissemination, including the capsular polysaccharide, mannitol, the mating type, melanin, phenotypic switching, phospholipase, prostaglandins and urease.Citation11 Several virulence factors have also been identified to play an important role in the transversal of Cryptococcus across the BBB and brain infection. Fungal cell morphology and capsule morphology have been reported to play a role in the BBB crossing. The production of giant/titan cells during infection prevents those cells from being engulfed by macrophages, thus cause ineffective dissemination from lung to the blood stream.Citation72 It is reasonable to speculate that the enlarged cells also could be more difficult to invade the BBB simply due to their size. The variation of Cryptococcus cell morphology referred as smooth, mucoid, or wrinkled form has been described.Citation89 The smooth C. gattii cells were reported to produce smaller capsules in general and are more efficient in crossing the BBB and causing CNS infection.Citation90
Cryptococcal urease has been found to contribute to dissemination to the central nervous system (CNS) following intravenous inoculation, but not the growth in the CNS.Citation78 Further study using real-time imaging microscopy showed that urease does not affect the trapping of the yeast in the capillary or the replication in the brain, but plays a role in the transmigration of yeast cells.Citation75 It was proposed that the product of urease, ammonia, might introduce a local damage of the endothelium, thus increasing permeability and leading to transmigration of Cryptococcus.Citation75
Fungal inositol transporters have also been found to play a role in cryptococcal virulence, including brain infection.Citation19,Citation91,Citation92 Several factors point to inositol as a potential host factor promoting the development of cryptococcal meningitis. First, both human and animal brains contain abundant free inositol, which plays a critical role in the normality of neurological response and psychological feedbacks.Citation93,Citation94 Inositol is a major osmolyte in the human and animal brains. It is present in the human cerebellum at over 200-fold higher concentrations than found in plasma.Citation93 Second, Cryptococcus can utilize inositol as a sole carbon source, which may provide a growth advantage during brain infection.Citation95,Citation96 Evidence from pathological studies showed that Cryptococcus cells associate with inositol-rich microglial cells during CNS cryptococcosis.Citation97,Citation98 Third, an unusually large inositol transporter gene family with over ten members has been identified in Cryptococcus, and they appear to play an important role in fungal virulence.Citation19,Citation91,Citation92 Mutants lacking two major fungal inositol transporters Itr1a and Itr3c showed attenuated virulence in several murine models (intranasal inhalation model, intravenous injection model and intracerebral injection model), indicating the fungal inositol acquisition system is required for Cryptococcus-host interaction, particularly during brain infection. Recently, we found that inositol can directly increase the rate of Cryptococcus transversal across the BBB in an in vitro BBB model, and the inositol effect is fungal inositol transporter-dependent (our unpublished results). This discovery suggests that inositol sensing and utilization could be another important virulence factor for development of cryptococcal meningitis.
The cryptococcal inositol phosphosphingolipid phospholipase C1 gene (Isc1) has also been shown to be important for controlling the dissemination of C. neoformans to the brain in mice. The isc1Δ mutant produces hyperencapsulated cells and fails to invade brain to cause CNS cryptococcosis.Citation67 This also suggests that capsule size is important for fungal dissemination to the CNS and the development of C. neoformans meningoencephalitis. Phosphatidylinositol 4-kinase (Pik1), an enzyme involved in inositol metabolism to produce phosphatidylinositol 4-phosphate, has been reported to be essential for fungal survival on medium containing cerebrospinal fluid (CSF) in vitro and during brain infection in a rabbit meningitis model in vivo.Citation99
The Cryptococcus Plb1 has been reported to be secreted in a phosphatidylinositol transfer protein Sec14-dependent manner.Citation100 Both in vitro and in vivo experiments have established that Plb1 facilitates adherence to lung epithelium, establishment of interstitial lung infection, survival and replication of Cryptococcus within macrophages, and dissemination to the CNS.Citation70,Citation101,Citation102 A defect in dissemination of plb1Δ mutants may be due to the reduced ability for yeast cells to escape from macrophages, a process commonly called vomocytosis.Citation100
Lastly, a number of regulatory factors, including the sterol regulatory element-binding protein (Sre1)Citation103 and copper-dependent transcription factor 1 (Cuf1),Citation104 contribute to the infection of C. neoformans in the brain. Mice infected by the sre1Δ mutant showed an accumulation of yeast cells in the meningeal layer but no cystic lesion development in the brain, suggesting that Sre1 is important for adaptation and growth in the brain but is dispensable for invasion into the brain.Citation103 Meanwhile, Cuf1 was found to play a role in the dissemination of cryptococcal cells to the brain, but not their proliferation in the lung based on mouse models. Cuf1 regulates the expression of the low affinity copper transporter Ctr4 in C. neoformans. The expression level of CTR4 is upregulated during brain infection and its expression pattern in patients with systemic cryptococcosis is highly correlated with Cryptococcus growth in the brain.Citation104 These observations suggest that copper acquisition is important for fungal pathogenesis during neurologic infection.
Animal Models for Cryptococcal Meningitis
Besides causing human infection, Cryptococcus is also a wide-range veterinary pathogen that naturally infects cats, dogs, cows, horses and primates, as well as some invertebrate species. The similarity of disease infections and clinical manifestation between human and animals has led to the development of several excellent animal models for cryptococcosis, including rabbit, mouse, guinea pig and rat.Citation105 The guinea pig was the first animal model system selected for the study of cryptococcosis,Citation106 and the model has been improved for antifungal therapy study. Its medium body size makes it a good chronic infection model for antifungal therapy studies.Citation107 The disadvantage is that the nature of the immune response is not well studied, and only a few inbred guinea pig strains exist. Rabbits are naturally resistant to cryptococcal infection, and immune suppression with corticosteroids is commonly used to circumvent intrinsic host resistance. The strong correlation between rabbit response to antifungal treatment and human disease outcome makes the rabbit a valuable model host for cryptococcal meningitis.Citation108,Citation109 The large size of rabbits allows for frequent, repetitive sampling of CSF, facilitating the investigation of chronic cryptococcal meningitis.Citation109 A potential limitation of the rabbit model is the availability of only a few inbred strains, few molecular tools and the requirement for immune suppression. The mouse is the most popular model host for laboratory study of cryptococcal infection. The mouse intranasal inhalation model mimics the natural route of cryptococcal infection. The mouse intravenous injection model directly deposits fungal cells in the blood circulation and is commonly used for testing the BBB crossing, while the mouse intracerebral injection model is used for studying the development of CNS cryptococcosis. The major advantage of this model is its relatively low cost, ease of handling, the availability of numerous inbred strains with well-developed tools for immunologic and genetic investigation and the ability to produce a clinically relevant experimental cryptococcal infection.Citation110 Rats have features common to mice, but its relative large size is advantageous for the experimental procedures such as intratracheal infection or sampling of CSF.Citation111
Meningitis Caused by Other Fungi
Besides cryptococcal meningitis, several other medically important fungi can also infect brain to cause meningitis with high mortality. Most fungi causing CNS infection are saprobes with worldwide distribution; a few are geographically restricted like Coccidioides immitis.Citation112 Among them, CNS infection by the dimorphic fungi Histoplasma capsulatum or C. immitis is well described but an uncommon manifestation of disseminated disease. Other medically important fungi, including Aspergillus spp,Citation113 Candida spp, Blastomyces dermatitidis, Paracoccidioides brasiliensis, as well as Zygomycetes can also infrequently cause meningitisCitation10 (). In addition, a subgroup of the dematiaceous molds (Phaeohyphomycoses), such as Bipolaris spicifera, Exophiala jeanselmei, Cladophialophora bantiana, Ochroconis gallopavum and Ramichloridium mackenziei, also have been reported to cause often lethal CNS infection even in immunocompetent patients.Citation8,Citation114,Citation115 The fact that these dematiaceous molds and Cryptococcus both produce dark brown melanin and both have CNS tropism is an interesting correlation. It would be interesting to investigate whether there is any commonality in the molecular mechanism of brain invasion among these fungi.
Compared with cryptococcal meningitis, mechanistic understanding of the disease development in other fungal meningitis remains limited. It has been reported that the transcellular mechanism is used for transversal of Candida spp across the BBB.Citation116 Candida invasion of brain endothelial cells has been shown to be mediated by the fungal invasins, Als3 and Ssa1, through interacting with host cell receptor gp96.Citation117 H. capsulatum cell surface protein Yps3 has been reported to play a role in fungal transition and pathogenicity.Citation118 This protein has also been found to induce Toll-like receptor 2 (TLR2) signaling, including the activation of NFκB in microglial cells, indicating that Yps3 plays an important role in Histoplasma CNS infection.Citation119 Very few experimental data have been established on how other fungi invade brain. Meanwhile, current strategies on disease epidemiology, diagnosis, and antifungal therapy of fungal meningitis caused by these fungi have been well documented in a number of excellent reviews.Citation9,Citation10,Citation112,Citation115,Citation120
Concluding Remarks and Future Perspective
Despite awareness of HIV/AIDS and the application of widespread antiretroviral therapy that reduced overall opportunistic infections in AIDS, the mortality and morbidity rate caused by fungal meningitis remains unacceptably high, especially in resource-limited certain regions of the world. Also, the advance of modern medical interventions, such as cancer chemotherapy, organ transplantation, and other therapies, have increased immunocompromised populations without HIV-1 infection that are also susceptible to opportunistic fungal infections. Fungal meningitis remains a serious medical problem even in the countries with advanced healthcare systems. Molecular studies on host-pathogen interactions during systemic fungal infections remain an urgent priority to better understand disease mechanisms that will help formulate better disease control strategies.
Acknowledgments
We thank Issar Smith, Arturo Casadevall and Chun Chang for critical reading of the manuscript and valuable comments. This work was supported by NIH grant AI069397 to D.S.P. and American Heart Association grant 12SDG911034 and UMDNJ institutional start-up funds to C.X.
References
- Honda H, Warren DK. Central nervous system infections: meningitis and brain abscess. Infect Dis Clin North Am 2009; 23:609 - 23; http://dx.doi.org/10.1016/j.idc.2009.04.009; PMID: 19665086
- van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med 2006; 354:44 - 53; http://dx.doi.org/10.1056/NEJMra052116; PMID: 16394301
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41 - 53; http://dx.doi.org/10.1038/nrn1824; PMID: 16371949
- Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol 2008; 6:625 - 34; http://dx.doi.org/10.1038/nrmicro1952; PMID: 18604221
- Kim KS. Microbial translocation of the blood-brain barrier. Int J Parasitol 2006; 36:607 - 14; http://dx.doi.org/10.1016/j.ijpara.2006.01.013; PMID: 16542662
- Tuomanen E. Entry of pathogens into the central nervous system. FEMS Microbiol Rev 1996; 18:289 - 99; http://dx.doi.org/10.1111/j.1574-6976.1996.tb00245.x; PMID: 8703507
- Klein BS, Tebbets B. Dimorphism and virulence in fungi. Curr Opin Microbiol 2007; 10:314 - 9; http://dx.doi.org/10.1016/j.mib.2007.04.002; PMID: 17719267
- Naggie S, Perfect JR. Molds: hyalohyphomycosis, phaeohyphomycosis, and zygomycosis. [vii-viii.] Clin Chest Med 2009; 30:337 - 53; http://dx.doi.org/10.1016/j.ccm.2009.02.009; PMID: 19375639
- Scully EP, Baden LR, Katz JT. Fungal brain infections. Curr Opin Neurol 2008; 21:347 - 52; http://dx.doi.org/10.1097/WCO.0b013e3282fee95b; PMID: 18451721
- Gottfredsson M, Perfect JR. Fungal meningitis. Semin Neurol 2000; 20:307 - 22; http://dx.doi.org/10.1055/s-2000-9394; PMID: 11051295
- Perfect JR, Casadevall A. Cryptococcosis. [v-vi.] Infect Dis Clin North Am 2002; 16:837 - 74; http://dx.doi.org/10.1016/S0891-5520(02)00036-3; PMID: 12512184
- Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009; 23:525 - 30; http://dx.doi.org/10.1097/QAD.0b013e328322ffac; PMID: 19182676
- Powderly WG. Cryptococcal Meningitis in HIV-Infected Patients. Curr Infect Dis Rep 2000; 2:352 - 7; http://dx.doi.org/10.1007/s11908-000-0015-y; PMID: 11095877
- Sanfelice F. Contributo alle morfologia e biologia dei blastomiceti che si sviluppano nei succhi di alcuni frutti. Ann Ig 1894; 4:463 - 95
- Kwon-Chung KJ, Bennett JE. Epidemiologic differences between the two varieties of Cryptococcus neoformans. Am J Epidemiol 1984; 120:123 - 30; PMID: 6377880
- Ellis D, Pfeiffer T. The ecology of Cryptococcus neoformans. Eur J Epidemiol 1992; 8:321 - 5; http://dx.doi.org/10.1007/BF00158562; PMID: 1397195
- Pfeiffer TJ, Ellis DH. Environmental isolation of Cryptococcus neoformans var. gattii from Eucalyptus tereticornis. J Med Vet Mycol 1992; 30:407 - 8; http://dx.doi.org/10.1080/02681219280000541; PMID: 1469544
- Litvintseva AP, Xu J, Mitchell TG. Population structure and ecology of Cryptococcus noformans and Cryptococcus gattii. In: Heitman J, Kozel., T. R., Kwon-Chung, K. J., Perfect, J. R., and Casadevall, A., ed. Cryptococcus: from human pathogen to model yeast. Washington D. C.: ASM Press, 2011:P97-111.
- Xue C, Tada Y, Dong X, Heitman J. The human fungal pathogen Cryptococcus can complete its sexual cycle during a pathogenic association with plants. Cell Host Microbe 2007; 1:263 - 73; http://dx.doi.org/10.1016/j.chom.2007.05.005; PMID: 18005707
- Nielsen K, De Obaldia AL, Heitman J. Cryptococcus neoformans mates on pigeon guano: implications for the realized ecological niche and globalization. Eukaryot Cell 2007; 6:949 - 59; http://dx.doi.org/10.1128/EC.00097-07; PMID: 17449657
- Kidd SE, Hagen F, Tscharke RL, Huynh M, Bartlett KH, Fyfe M, et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proc Natl Acad Sci USA 2004; 101:17258 - 63; http://dx.doi.org/10.1073/pnas.0402981101; PMID: 15572442
- Byrnes EJ 3rd, Li W, Lewit Y, Ma H, Voelz K, Ren P, et al. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog 2010; 6:e1000850; http://dx.doi.org/10.1371/journal.ppat.1000850; PMID: 20421942
- Kronstad JW, Attarian R, Cadieux B, Choi J, D'Souza CA, Griffiths EJ, et al. Expanding fungal pathogenesis: Cryptococcus breaks out of the opportunistic box. Nat Rev Microbiol 2011; 9:193 - 203; http://dx.doi.org/10.1038/nrmicro2522; PMID: 21326274
- Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, et al. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 2005; 437:1360 - 4; http://dx.doi.org/10.1038/nature04220; PMID: 16222245
- Byrnes EJ 3rd, Li W, Lewit Y, Perfect JR, Carter DA, Cox GM, et al. First reported case of Cryptococcus gattii in the Southeastern USA: implications for travel-associated acquisition of an emerging pathogen. PLoS ONE 2009; 4:e5851; http://dx.doi.org/10.1371/journal.pone.0005851; PMID: 19516904
- Velagapudi R, Hsueh YP, Geunes-Boyer S, Wright JR, Heitman J. Spores as infectious propagules of Cryptococcus neoformans. Infect Immun 2009; 77:4345 - 55; http://dx.doi.org/10.1128/IAI.00542-09; PMID: 19620339
- Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect Immun 2009; 77:3491 - 500; http://dx.doi.org/10.1128/IAI.00334-09; PMID: 19451235
- Hatch TF. Distribution and deposition of inhaled particles in respiratory tract. Bacteriol Rev 1961; 25:237 - 40; PMID: 13905321
- Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol 1999; 37:3204 - 9; PMID: 10488178
- Chen LC, Goldman DL, Doering TL, Pirofski L, Casadevall A. Antibody response to Cryptococcus neoformans proteins in rodents and humans. Infect Immun 1999; 67:2218 - 24; PMID: 10225877
- Eisenman HC, Casadevall A, McClelland EE. New insights on the pathogenesis of invasive Cryptococcus neoformans infection. Curr Infect Dis Rep 2007; 9:457 - 64; http://dx.doi.org/10.1007/s11908-007-0070-8; PMID: 17999881
- Feldmesser M, Kress Y, Casadevall A. Intracellular crystal formation as a mechanism of cytotoxicity in murine pulmonary Cryptococcus neoformans infection. Infect Immun 2001; 69:2723 - 7; http://dx.doi.org/10.1128/IAI.69.4.2723-2727.2001; PMID: 11254641
- Casadevall A, Perfect JR. Cryptococcus neoformans. Washington, DC: ASM Press, 1998.
- Huffnagle GB, Lipscomb MF. Cells and cytokines in pulmonary cryptococcosis. Res Immunol 1998; 149:387 - 96, discussion 512-4; http://dx.doi.org/10.1016/S0923-2494(98)80762-1; PMID: 9720956
- Lortholary O, Improvisi L, Rayhane N, Gray F, Fitting C, Cavaillon JM, et al. Cytokine profiles of AIDS patients are similar to those of mice with disseminated Cryptococcus neoformans infection. Infect Immun 1999; 67:6314 - 20; PMID: 10569743
- Feldmesser M, Casadevall A. Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol 1997; 158:790 - 9; PMID: 8992996
- Alvarez M, Casadevall A. Phagosome extrusion and host-cell survival after Cryptococcus neoformans phagocytosis by macrophages. Curr Biol 2006; 16:2161 - 5; http://dx.doi.org/10.1016/j.cub.2006.09.061; PMID: 17084702
- Alvarez M, Casadevall A. Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages. BMC Immunol 2007; 8:16; http://dx.doi.org/10.1186/1471-2172-8-16; PMID: 17705844
- Ma H, Croudace JE, Lammas DA, May RC. Expulsion of live pathogenic yeast by macrophages. Curr Biol 2006; 16:2156 - 60; http://dx.doi.org/10.1016/j.cub.2006.09.032; PMID: 17084701
- Johnston SA, May RC. The human fungal pathogen Cryptococcus neoformans escapes macrophages by a phagosome emptying mechanism that is inhibited by Arp2/3 complex-mediated actin polymerisation. PLoS Pathog 2010; 6:e1001041; http://dx.doi.org/10.1371/journal.ppat.1001041; PMID: 20714349
- Chang YC, Stins MF, McCaffery MJ, Miller GF, Pare DR, Dam T, et al. Cryptococcal yeast cells invade the central nervous system via transcellular penetration of the blood-brain barrier. Infect Immun 2004; 72:4985 - 95; http://dx.doi.org/10.1128/IAI.72.9.4985-4995.2004; PMID: 15321990
- Kraus PR, Nichols CB, Heitman J. Calcium- and calcineurin-independent roles for calmodulin in Cryptococcus neoformans morphogenesis and high-temperature growth. Eukaryot Cell 2005; 4:1079 - 87; http://dx.doi.org/10.1128/EC.4.6.1079-1087.2005; PMID: 15947200
- Gerik KJ, Donlin MJ, Soto CE, Banks AM, Banks IR, Maligie MA, et al. Cell wall integrity is dependent on the PKC1 signal transduction pathway in Cryptococcus neoformans. Mol Microbiol 2005; 58:393 - 408; http://dx.doi.org/10.1111/j.1365-2958.2005.04843.x; PMID: 16194228
- Gerik KJ, Bhimireddy SR, Ryerse JS, Specht CA, Lodge JK. PKC1 is essential for protection against both oxidative and nitrosative stresses, cell integrity, and normal manifestation of virulence factors in the pathogenic fungus Cryptococcus neoformans. Eukaryot Cell 2008; 7:1685 - 98; http://dx.doi.org/10.1128/EC.00146-08; PMID: 18689526
- Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans. Regulation of capsule synthesis by carbon dioxide. J Clin Invest 1985; 76:508 - 16; http://dx.doi.org/10.1172/JCI112000; PMID: 3928681
- Bahn YS, Cox GM, Perfect JR, Heitman J. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol 2005; 15:2013 - 20; http://dx.doi.org/10.1016/j.cub.2005.09.047; PMID: 16303560
- Chrisman CJ, Albuquerque P, Guimaraes AJ, Nieves E, Casadevall A. Phospholipids trigger Cryptococcus neoformans capsular enlargement during interactions with amoebae and macrophages. PLoS Pathog 2011; 7:e1002047; http://dx.doi.org/10.1371/journal.ppat.1002047; PMID: 21637814
- Jung WH, Sham A, White R, Kronstad JW. Iron regulation of the major virulence factors in the AIDS-associated pathogen Cryptococcus neoformans. PLoS Biol 2006; 4:e410; http://dx.doi.org/10.1371/journal.pbio.0040410; PMID: 17121456
- Syme RM, Bruno TF, Kozel TR, Mody CH. The capsule of Cryptococcus neoformans reduces T-lymphocyte proliferation by reducing phagocytosis, which can be restored with anticapsular antibody. Infect Immun 1999; 67:4620 - 7; PMID: 10456908
- Yauch LE, Lam JS, Levitz SM. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS Pathog 2006; 2:e120; http://dx.doi.org/10.1371/journal.ppat.0020120; PMID: 17096589
- Pericolini E, Cenci E, Monari C, De Jesus M, Bistoni F, Casadevall A, et al. Cryptococcus neoformans capsular polysaccharide component galactoxylomannan induces apoptosis of human T-cells through activation of caspase-8. Cell Microbiol 2006; 8:267 - 75; http://dx.doi.org/10.1111/j.1462-5822.2005.00619.x; PMID: 16441437
- Oscarson S, Alpe M, Svahnberg P, Nakouzi A, Casadevall A. Synthesis and immunological studies of glycoconjugates of Cryptococcus neoformans capsular glucuronoxylomannan oligosaccharide structures. Vaccine 2005; 23:3961 - 72; http://dx.doi.org/10.1016/j.vaccine.2005.02.029; PMID: 15917118
- Vecchiarelli A, Pericolini E, Gabrielli E, Chow SK, Bistoni F, Cenci E, et al. Cryptococcus neoformans galactoxylomannan is a potent negative immunomodulator, inspiring new approaches in anti-inflammatory immunotherapy. Immunotherapy 2011; 3:997 - 1005; http://dx.doi.org/10.2217/imt.11.86; PMID: 21843086
- Vecchiarelli A. Immunoregulation by capsular components of Cryptococcus neoformans.. Med Mycol 2000; 38:407 - 17; PMID: 11204878
- Doering TL, Nosanchuk JD, Roberts WK, Casadevall A. Melanin as a potential cryptococcal defence against microbicidal proteins. Med Mycol 1999; 37:175 - 81; PMID: 10421849
- Nosanchuk JD, Casadevall A. The contribution of melanin to microbial pathogenesis. Cell Microbiol 2003; 5:203 - 23; http://dx.doi.org/10.1046/j.1462-5814.2003.00268.x; PMID: 12675679
- Zhu X, Williamson PR. Role of laccase in the biology and virulence of Cryptococcus neoformans. FEMS Yeast Res 2004; 5:1 - 10; http://dx.doi.org/10.1016/j.femsyr.2004.04.004; PMID: 15381117
- Noverr MC, Williamson PR, Fajardo RS, Huffnagle GB. CNLAC1 is required for extrapulmonary dissemination of Cryptococcus neoformans but not pulmonary persistence. Infect Immun 2004; 72:1693 - 9; http://dx.doi.org/10.1128/IAI.72.3.1693-1699.2004; PMID: 14977977
- Bahn YS, Xue C, Idnurm A, Rutherford JC, Heitman J, Cardenas ME. Sensing the environment: lessons from fungi. Nat Rev Microbiol 2007; 5:57 - 69; http://dx.doi.org/10.1038/nrmicro1578; PMID: 17170747
- Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev 1997; 11:3206 - 17; http://dx.doi.org/10.1101/gad.11.23.3206; PMID: 9389652
- Luberto C, Toffaletti DL, Wills EA, Tucker SC, Casadevall A, Perfect JR, et al. Roles for inositol-phosphoryl ceramide synthase 1 (IPC1) in pathogenesis of C. neoformans. Genes Dev 2001; 15:201 - 12; http://dx.doi.org/10.1101/gad.856001; PMID: 11157776
- Shea JM, Del Poeta M. Lipid signaling in pathogenic fungi. Curr Opin Microbiol 2006; 9:352 - 8; http://dx.doi.org/10.1016/j.mib.2006.06.003; PMID: 16798065
- Heung LJ, Luberto C, Plowden A, Hannun YA, Del Poeta M. The sphingolipid pathway regulates Pkc1 through the formation of diacylglycerol in Cryptococcus neoformans. J Biol Chem 2004; 279:21144 - 53; http://dx.doi.org/10.1074/jbc.M312995200; PMID: 15014071
- Mare L, Iatta R, Montagna MT, Luberto C, Del Poeta M. APP1 transcription is regulated by inositol-phosphorylceramide synthase 1-diacylglycerol pathway and is controlled by ATF2 transcription factor in Cryptococcus neoformans. J Biol Chem 2005; 280:36055 - 64; http://dx.doi.org/10.1074/jbc.M507285200; PMID: 16129666
- Heung LJ, Kaiser AE, Luberto C, Del Poeta M. The role and mechanism of diacylglycerol-protein kinase C1 signaling in melanogenesis by Cryptococcus neoformans. J Biol Chem 2005; 280:28547 - 55; http://dx.doi.org/10.1074/jbc.M503404200; PMID: 15946943
- Luberto C, Martinez-Marino B, Taraskiewicz D, Bolanos B, Chitano P, Toffaletti DL, et al. Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J Clin Invest 2003; 112:1080 - 94; PMID: 14523045
- Shea JM, Kechichian TB, Luberto C, Del Poeta M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun 2006; 74:5977 - 88; http://dx.doi.org/10.1128/IAI.00768-06; PMID: 16988277
- Chen SC, Wright LC, Golding JC, Sorrell TC. Purification and characterization of secretory phospholipase B, lysophospholipase and lysophospholipase/transacylase from a virulent strain of the pathogenic fungus Cryptococcus neoformans.. Biochem J 2000; 347:431 - 9; http://dx.doi.org/10.1042/0264-6021:3470431; PMID: 10749672
- Wright LC, Santangelo RM, Ganendren R, Payne J, Djordjevic JT, Sorrell TC. Cryptococcal lipid metabolism: phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot Cell 2007; 6:37 - 47; http://dx.doi.org/10.1128/EC.00262-06; PMID: 17099081
- Santangelo R, Zoellner H, Sorrell T, Wilson C, Donald C, Djordjevic J, et al. Role of extracellular phospholipases and mononuclear phagocytes in dissemination of cryptococcosis in a murine model. Infect Immun 2004; 72:2229 - 39; http://dx.doi.org/10.1128/IAI.72.4.2229-2239.2004; PMID: 15039347
- Nielsen K, Cox GM, Litvintseva AP, Mylonakis E, Malliaris SD, Benjamin DK Jr., et al. Cryptococcus neoformans {alpha} strains preferentially disseminate to the central nervous system during coinfection. Infect Immun 2005; 73:4922 - 33; http://dx.doi.org/10.1128/IAI.73.8.4922-4933.2005; PMID: 16041006
- Okagaki LH, Strain AK, Nielsen JN, Charlier C, Baltes NJ, Chretien F, et al. Cryptococcal cell morphology affects host cell interactions and pathogenicity. PLoS Pathog 2010; 6:e1000953; http://dx.doi.org/10.1371/journal.ppat.1000953; PMID: 20585559
- Zaragoza O, Garcia-Rodas R, Nosanchuk JD, Cuenca-Estrella M, Rodriguez-Tudela JL, Casadevall A. Fungal cell gigantism during mammalian infection. PLoS Pathog 2010; 6:e1000945; http://dx.doi.org/10.1371/journal.ppat.1000945; PMID: 20585557
- Okagaki LH, Wang Y, Ballou ER, O'Meara TR, Bahn YS, Alspaugh JA, et al. Cryptococcal titan cell formation is regulated by G-protein signaling in response to multiple stimuli. Eukaryot Cell 2011.
- Shi M, Li SS, Zheng C, Jones GJ, Kim KS, Zhou H, et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest 2010; 120:1683 - 93; http://dx.doi.org/10.1172/JCI41963; PMID: 20424328
- Chrétien F, Lortholary O, Kansau I, Neuville S, Gray F, Dromer F. Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis 2002; 186:522 - 30; http://dx.doi.org/10.1086/341564; PMID: 12195380
- Charlier C, Nielsen K, Daou S, Brigitte M, Chretien F, Dromer F. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun 2009; 77:120 - 7; http://dx.doi.org/10.1128/IAI.01065-08; PMID: 18936186
- Olszewski MA, Noverr MC, Chen GH, Toews GB, Cox GM, Perfect JR, et al. Urease expression by Cryptococcus neoformans promotes microvascular sequestration, thereby enhancing central nervous system invasion. Am J Pathol 2004; 164:1761 - 71; http://dx.doi.org/10.1016/S0002-9440(10)63734-0; PMID: 15111322
- Charlier C, Chretien F, Baudrimont M, Mordelet E, Lortholary O, Dromer F. Capsule structure changes associated with Cryptococcus neoformans crossing of the blood-brain barrier. Am J Pathol 2005; 166:421 - 32; http://dx.doi.org/10.1016/S0002-9440(10)62265-1; PMID: 15681826
- Chen SH, Stins MF, Huang SH, Chen YH, Kwon-Chung KJ, Chang Y, et al. Cryptococcus neoformans induces alterations in the cytoskeleton of human brain microvascular endothelial cells. J Med Microbiol 2003; 52:961 - 70; http://dx.doi.org/10.1099/jmm.0.05230-0; PMID: 14532340
- Vu K, Weksler B, Romero I, Couraud PO, Gelli A. Immortalized human brain endothelial cell line HCMEC/D3 as a model of the blood-brain barrier facilitates in vitro studies of central nervous system infection by Cryptococcus neoformans.. Eukaryot Cell 2009; 8:1803 - 7; http://dx.doi.org/10.1128/EC.00240-09; PMID: 19767445
- Jong A, Wu CH, Shackleford GM, Kwon-Chung KJ, Chang YC, Chen HM, et al. Involvement of human CD44 during Cryptococcus neoformans infection of brain microvascular endothelial cells. Cell Microbiol 2008; 10:1313 - 26; http://dx.doi.org/10.1111/j.1462-5822.2008.01128.x; PMID: 18248627
- Huang SH, Long M, Wu CH, Kwon-Chung KJ, Chang YC, Chi F, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells is mediated through the lipid rafts-endocytic pathway via the dual specificity tyrosine-phosphorylation-regulated kinase 3 (DYRK3). J Biol Chem 2011.
- Jong A, Wu CH, Prasadarao NV, Kwon-Chung KJ, Chang YC, Ouyang Y, et al. Invasion of Cryptococcus neoformans into human brain microvascular endothelial cells requires protein kinase C-alpha activation. Cell Microbiol 2008; 10:1854 - 65; http://dx.doi.org/10.1111/j.1462-5822.2008.01172.x; PMID: 18489726
- Feldmesser M, Tucker S, Casadevall A. Intracellular parasitism of macrophages by Cryptococcus neoformans. Trends Microbiol 2001; 9:273 - 8; http://dx.doi.org/10.1016/S0966-842X(01)02035-2; PMID: 11390242
- Voelz K, May RC. Cryptococcal interactions with the host immune system. Eukaryot Cell 2010; 9:835 - 46; http://dx.doi.org/10.1128/EC.00039-10; PMID: 20382758
- Feldmesser M, Kress Y, Novikoff P, Casadevall A. Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 2000; 68:4225 - 37; http://dx.doi.org/10.1128/IAI.68.7.4225-4237.2000; PMID: 10858240
- Kechichian TB, Shea J, Del Poeta M. Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun 2007; 75:4792 - 8; http://dx.doi.org/10.1128/IAI.00587-07; PMID: 17664261
- Guerrero A, Jain N, Goldman DL, Fries BC. Phenotypic switching in Cryptococcus neoformans. Microbiology 2006; 152:3 - 9; http://dx.doi.org/10.1099/mic.0.28451-0; PMID: 16385110
- Jain N, Guerrero A, Fries BC. Phenotypic switching and its implications for the pathogenesis of Cryptococcus neoformans. FEMS Yeast Res 2006; 6:480 - 8; http://dx.doi.org/10.1111/j.1567-1364.2006.00039.x; PMID: 16696644
- Xue C, Liu T, Li W, Liu I, Kronstad JW, Seyfang A, et al. Role of an expanded inositol transporter repertoire in Cryptococcus neoformans sexual reproduction and virulence. mBio 2010; 1:e00084-10.
- Wang Y, Liu TB, Delmas G, Park S, Perlin D, Xue C. Two major inositol transporters and their role in cryptococcal virulence. Eukaryot Cell 2011; 10:618 - 28; http://dx.doi.org/10.1128/EC.00327-10; PMID: 21398509
- Fisher SK, Novak JE, Agranoff BW. Inositol and higher inositol phosphates in neural tissues: homeostasis, metabolism and functional significance. J Neurochem 2002; 82:736 - 54; http://dx.doi.org/10.1046/j.1471-4159.2002.01041.x; PMID: 12358779
- Haris M, Cai K, Singh A, Hariharan H, Reddy R. In vivo mapping of brain myo-inositol. Neuroimage 2011; 54:2079 - 85; http://dx.doi.org/10.1016/j.neuroimage.2010.10.017; PMID: 20951217
- Barnett JA. The utilization of sugars by yeasts. Adv Carbohydr Chem Biochem 1976; 32:125 - 234; http://dx.doi.org/10.1016/S0065-2318(08)60337-6; PMID: 782183
- Healy ME, Dillavou CL, Taylor GE. Diagnostic medium containing inositol, urea, and caffeic acid for selective growth of Cryptococcus neoformans. J Clin Microbiol 1977; 6:387 - 91; PMID: 334795
- Lee SC, Dickson DW, Casadevall A. Pathology of cryptococcal meningoencephalitis: analysis of 27 patients with pathogenetic implications. Hum Pathol 1996; 27:839 - 47; http://dx.doi.org/10.1016/S0046-8177(96)90459-1; PMID: 8760020
- Lee SC, Casadevall A, Dickson DW. Immunohistochemical localization of capsular polysaccharide antigen in the central nervous system cells in cryptococcal meningoencephalitis. Am J Pathol 1996; 148:1267 - 74; PMID: 8644867
- Lee A, Toffaletti DL, Tenor J, Soderblom EJ, Thompson JW, Moseley MA, et al. Survival defects of Cryptococcus neoformans mutants exposed to human cerebrospinal fluid result in attenuated virulence in an experimental model of meningitis. Infect Immun 2010; 78:4213 - 25; http://dx.doi.org/10.1128/IAI.00551-10; PMID: 20696827
- Chayakulkeeree M, Johnston SA, Oei JB, Lev S, Williamson PR, Wilson CF, et al. SEC14 is a specific requirement for secretion of phospholipase B1 and pathogenicity of Cryptococcus neoformans. Mol Microbiol 2011; 80:1088 - 101; http://dx.doi.org/10.1111/j.1365-2958.2011.07632.x; PMID: 21453402
- Cox GM, McDade HC, Chen SC, Tucker SC, Gottfredsson M, Wright LC, et al. Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol 2001; 39:166 - 75; http://dx.doi.org/10.1046/j.1365-2958.2001.02236.x; PMID: 11123698
- Noverr MC, Cox GM, Perfect JR, Huffnagle GB. Role of PLB1 in pulmonary inflammation and cryptococcal eicosanoid production. Infect Immun 2003; 71:1538 - 47; http://dx.doi.org/10.1128/IAI.71.3.1538-1547.2003; PMID: 12595473
- Chang YC, Bien CM, Lee H, Espenshade PJ, Kwon-Chung KJ. Sre1p, a regulator of oxygen sensing and sterol homeostasis, is required for virulence in Cryptococcus neoformans. Mol Microbiol 2007; 64:614 - 29; http://dx.doi.org/10.1111/j.1365-2958.2007.05676.x; PMID: 17462012
- Waterman SR, Hacham M, Hu G, Zhu X, Park YD, Shin S, et al. Role of a CUF1/CTR4 copper regulatory axis in the virulence of Cryptococcus neoformans.. J Clin Invest 2007; 117:794 - 802; http://dx.doi.org/10.1172/JCI30006; PMID: 17290306
- Carroll SF, Guillot L, Qureshi ST. Mammalian model hosts of cryptococcal infection. Comp Med 2007; 57:9 - 17; PMID: 17348287
- Riera CM, Masih DT, Nobile R. Experimental cryptococcosis in guinea pigs. Mycopathologia 1983; 82:179 - 84; http://dx.doi.org/10.1007/BF00439224; PMID: 6350885
- Kirkpatrick WR, Najvar LK, Bocanegra R, Patterson TF, Graybill JR. New guinea pig model of Cryptococcal meningitis. Antimicrob Agents Chemother 2007; 51:3011 - 3; http://dx.doi.org/10.1128/AAC.00085-07; PMID: 17562797
- Perfect JR, Lang SD, Durack DT. Chronic cryptococcal meningitis: a new experimental model in rabbits. Am J Pathol 1980; 101:177 - 94; PMID: 7004196
- Perfect JR. Rabbit model of cryptococcal meningitis. In: Zak O, Sande, M. A., ed. Handbook of animal models of infection. London: Academic Press, Inc, 1999:721-76.
- Lipscomb MF, Lyons CR, Izzo AA, Lovchik J, Wilder JA. Expermental pulmonary cryptococcal infection in mice. In: Zak O, Sande, M. A., ed. Handbook of animal models of infection. London: Academic Press, Inc., 1999:681-6.
- Goldman DL, Casadevall A, Cho Y, Lee SC. Cryptococcus neoformans meningitis in the rat. Lab Invest 1996; 75:759 - 70; PMID: 8973471
- Chakrabarti A. Epidemiology of central nervous system mycoses. Neurol India 2007; 55:191 - 7; http://dx.doi.org/10.4103/0028-3886.35679; PMID: 17921647
- Nadkarni T, Goel A. Aspergilloma of the brain: an overview. J Postgrad Med 2005; 51:Suppl 1 S37 - 41; PMID: 16519254
- Kantarcioglu AS, de Hoog GS. Infections of the central nervous system by melanized fungi: a review of cases presented between 1999 and 2004. Mycoses 2004; 47:4 - 13; http://dx.doi.org/10.1046/j.1439-0507.2003.00956.x; PMID: 14998393
- Perfect JR, Alexander BD, Schell WA. Phaeohyphomycoses. In: Kauffman CA, Pappas, P. G., Sobel, J. D., and Dismukes, W. E., ed. Essential of Clinical Mycology. New York: Springer 2011:305-17.
- Jong AY, Stins MF, Huang SH, Chen SH, Kim KS. Traversal of Candida albicans across human blood-brain barrier in vitro. Infect Immun 2001; 69:4536 - 44; http://dx.doi.org/10.1128/IAI.69.7.4536-4544.2001; PMID: 11401997
- Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG. Mechanisms of Candida albicans Trafficking to the Brain. PLoS Pathog 2011; 7:e1002305; http://dx.doi.org/10.1371/journal.ppat.1002305; PMID: 21998592
- Bohse ML, Woods JP. Surface localization of the Yps3p protein of Histoplasma capsulatum. Eukaryot Cell 2005; 4:685 - 93; http://dx.doi.org/10.1128/EC.4.4.685-693.2005; PMID: 15821128
- Aravalli RN, Hu S, Woods JP, Lokensgard JR. Histoplasma capsulatum yeast phase-specific protein Yps3p induces Toll-like receptor 2 signaling. J Neuroinflammation 2008; 5:30; http://dx.doi.org/10.1186/1742-2094-5-30; PMID: 18606009
- Denning DW, Hope WW. Therapy for fungal diseases: opportunities and priorities. Trends Microbiol 2010; 18:195 - 204; http://dx.doi.org/10.1016/j.tim.2010.02.004; PMID: 20207544