Abstract
Among bacteria that reach the central nervous system (CNS), Listeria monocytogenes (Lm) is one of deadliest, in human and ruminant. This facultative intracellular bacterium has the particularity to induce meningitis, meningoencephalitis and rhombencephalitis. Mechanisms by which Lm accesses the CNS remain poorly understood, but two major routes of infection have been proposed, based on clinical, in vitro and in vivo observations. A retrograde neural route is likely to occur in ruminants upon crossing of the oral epithelium, and this probably accounts for the observation that Lm induces almost exclusively rhombencephalitis in these animals. In contrast, the hematogenous route is likely the most frequent in human, in whom bacteria circulating in the blood, either free or associated with leukocytes are thought to breach the blood-brain barrier. New animal models that faithfully reproduce the hallmarks of human neurolisterisosis will allow addressing the molecular mechanisms underlying Lm ability to induce CNS disease, and improve our understanding of the pathophysiology of this deadly infection.
Introduction
Central nervous system (CNS) invasion by bacteria leads to severe infection, which can be fatal or associated with severe sequelae.Citation1,Citation2 Among the bacteria that can access the CNS, Listeria monocytogenes (Lm), a Gram-positive facultative intracellular bacterium, has the ability to induce meningitis and encephalitis.
The first known isolate of Lm was obtained from the cerebrospinal fluid (CSF) of a soldier who died from meningitis in 1918. The report was published in 1921,Citation3 before the characterization of a new bacterial species in 1926 from infected laboratory rabbits and guinea pigsCitation4 and in 1927 from wild gerbils.Citation5 This species is now known as Listeria monocytogenes. However, it is only during the second half of the 20th century that listeriosis started to be considered as a prominent human infection, concomitant with changes in food habits and the introduction of immunosuppressive therapies, in addition to being a well-characterized zoonosis routinely observed in domestic ruminants. The first human listeriosis outbreak linked to the absorption of contaminated food was published in 1983,Citation6 and Lm is now regarded as a classic foodborne pathogen for veterinarians and clinicians, which is well known to infect ruminants and humans via the oral route, and target similar organs: the central nervous system and the fetoplacental unit.Citation7
Even if listeriosis is rare in human (0.1 to 10 cases/million; 0.1% of all foodborne infections), it is considered as the most severe bacterial foodborne infection, responsible for 1,645 cases in Europe in 2009 (4 cases/million) for a case fatality rate of 16.6%Citation8 and around 2,500 cases per year in the USA, associated with a lethality of up to 30% in case of neurological involvement, even when appropriately treated.Citation9,Citation10 More than 50% of the cases correspond to septicemia, around 20–25% to CNS infections, 10–15% to maternal-fetal infections and the remaining to various localized infections (compiled data from the French National surveillance system in the past 5 years). After a decrease in the number of cases in the second part of the 20th century, which correlates with the implementation of controls in food industry and information campaigns directed to pregnant women,Citation11,Citation12 the incidence of Lm has slightly re-increased in recent years, notably in Europe.Citation8,Citation13-Citation16 The reason for this increase is unknown, but is likely a combination of the relative increase of the population at risk for listeriosis, such as the immunosuppressed host and/or elderly, and changes in food processing and habits.
The intracellular life of Lm and immune responses to this bacterium have been extensively studied in vitro and in vivo,Citation17,Citation18 yet many aspects of the pathophysiology of listeriosis, and particularly that of neurolisteriosis remain elusive, in part due to a relative lack of relevant and easy-to-handle animal model that reproduces all the hallmarks of the human disease (see below). Despite these limitations, epidemiological and clinical studies in human and ruminant as well as in vitro and in vivo mouse experimental infections, allow making a general picture of the putative mechanisms of Lm CNS invasion and infection. These studies suggest that diverse pathways could be used by Lm to gain access to the CNS and could include a retrograde neural route of invasion and crossing of the blood-brain barrier (BBB) by blood-borne bacteria.Citation19,Citation20 Yet, the molecular factors on both bacterial and host sides remain to be discovered, and new animal models recently developed will likely be instrumental to study and better understand the molecular mechanisms underlying neurolisteriosis (see below).
Natural History of Neurolisteriosis
Lm and L. ivanovii are the two species of the genus Listeria (that includes Lm, L. ivanovii, L. innocua, L. welschimeri, L. seeligeri, L. grayi, L. marthii and L. rocourtiae) that have been described as pathogenic.Citation7 L. ivanovii is almost exclusively recovered from ruminant, yet it can rarely infect humansCitation21 and has not been described to disseminate to the CNS, while Lm is the only one that leads to CNS infection, in humans and in domestic ruminants,Citation19,Citation20 as well as in wild or domestic species such as cervidae (fallow deerCitation22), camelidae (llamaCitation23), rodents (chinchilla and squirrelsCitation24) and to a lesser extent carnivores: felidae (cat and cougarCitation25,Citation26) and canidae (dog, fox and racoon dogCitation24,Citation27). Lm-associated CNS infection can manifest as meningitis, meningoencephalitis, brain abscesses and rhombencephalitis,Citation28 the latter being typically associated with this bacterium. The ability of Lm to cause both acute meningitis and brain parenchymal infection differentiates it from other bacteria frequently responsible for meningitis such as Streptococcus pneumoniae,Citation29Neisseria meningitidisCitation30 and Haemophilus influenzae,Citation31 and underlines a commonality with Mycobacterium tuberculosis.Citation32
Of note, CNS infection in both humans and ruminants classically does not occur in pregnant individuals (data from the French National surveillance system and ref. Citation20), suggesting that (1) pregnancy is not a predisposing factor for neurolisteriosis and (2) placental and brain infection mechanisms are likely different. Interestingly, other human pathogens with a mucosal portal of entry, such as the apicomplex Toxoplasma gondii,Citation33 the bacterium Treponema pallidumCitation34 and enteroviruses,Citation35 have in common, as Lm, to disseminate to the CNS and the fetoplacental unit.
Neurolisteriosis in humans
CNS infection manifests in human as meningitis and meningoencephalitis, which are the most frequent clinical presentations observed in human, followed by brain stem infection (rhombencephalitis) and brain abscesses.
Meningitis and meningoencephalitis
Meningitis is defined as an inflammation of meninges, the protective membranes covering the central nervous system at the brain parenchyma and spinal cord levels. They are composed of the dura mater, the arachnoid and the pia mater. Meninges contain the cerebrospinal fluid, which is located in the subarachnoidal space, and vessels and capillaries that enter the brain parenchyma ().
Figure 1. Blood-CSF and blood-brain barriers. From top to bottom: (A) Capillaries from the meningeal compartment, which represent part of the blood-CSF barrier. Dural endothelial cells are fenestrated, while endothelial cells from the subarachnoid space are joined by tight junctions. (B) Blood-brain barrier is made of endothelial cells joined by tight junctions. They are in close contact with astrocyte’s “feet” (astrocyte’s cell projection), which enable the differentiation of brain endothelial cell junctions. (C) Epithelial cells from the choroid plexus produce the cerebrospinal fluid (CSF) and isolate the fenestrated endothelium from the CSF, hence forming together with the meningeal endothelium the blood-CSF barrier. (Adapted from ref. Citation64.)
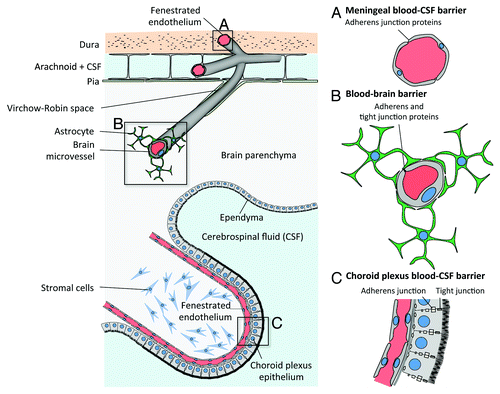
Meningoencephalitis is a brain inflammation associated with meningitis. Infection of the meninges might either occur upon the crossing of the blood-meningeal barriers or as a consequence of bacterial dissemination to and from the brain parenchyma.
Meningitis and meningoencephalitis account for the majority of CNS infection by Lm in human (70 to 97%).Citation36,Citation37 Lm has been recognized as the second to fourth cause of community-acquired acute bacterial meningitis in adults.Citation38,Citation39 Known predisposing factors are immunosuppression, age over 50 y and underlying conditions such as malignancy or diabetes.Citation36,Citation37
The clinical features of Lm meningitis differ from those of other bacterial meningitides in that Lm meningitis may have a sub-acute course (usually more than 24 h of symptoms before admission in hospital) and be associated with abnormal movements, seizures and alteration of consciousness. The onset of such symptoms is evocative of dissemination to the brain parenchyma and their association with meningeal signs of meningoencephalitis. In a recent prospective study on bacterial meningitis in adults,Citation36 Brouwer et al. compared meningitis due to Lm and to other bacterial meningitis. Only patients with a positive CSF bacterial culture were included. This study showed that the classic triad of fever, neck stiffness and change in mental status was present in 13 (43%) of 30 Lm-infected patients, which was not significantly different to what was observed for other bacterial meningitis in the same cohort.Citation39 However, typical cerebrospinal fluid findings predictive for bacterial meningitis were frequently absent: up to 23% of patients had no CSF abnormality indicative of bacterial meningitis, compared with only 12% for other bacterial meningitis. Gram staining of cerebrospinal fluid samples revealed the causative organism in only 24% of patients, compared with 80% for other bacterial meningitis. This result is in agreement with other studies showing that Gram staining of the CSF is often negative,Citation28 reflecting a quantitatively modest dissemination of Lm in the CSF. Besides clinical descriptions, few data are available on Lm-associated meningoencephalitis. CT scan resolution has proven so far to be insufficiently precise to gain insight into the neuropathology of Lm-associated encephalitis, and magnetic resonance imaging (MRI) allows visualization of diffuse but so far rather unspecific lesions.Citation40,Citation41
Brain abscess
In a small proportion of Lm CNS infections (up to 10%, with half of them in immunocompromised patients), macroscopic brain abscesses without meningeal involvement are observed.Citation42 Lm brain abscesses are revealed by imaging (CT scan and/or MRI) and are preferentially located in subcortical areas, thalamus, pons or medulla. These unusual locations have been reported as evocative of their listerial origin.Citation43,Citation44 Less than 10% of these patients have no known underlying condition. This disease is also sub-acute, and CSF cultures are positive in 25% of cases, indicative of concomitant and possibly secondary meningitis.
Rhombencephalitis
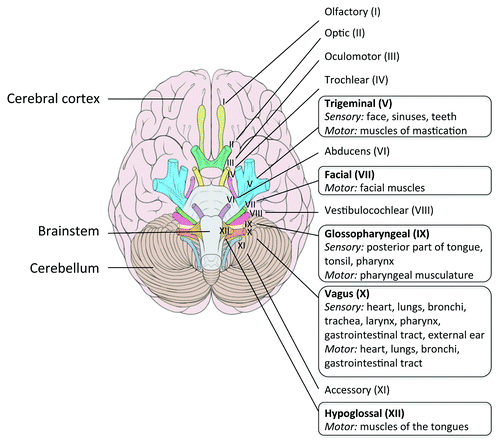
Rhombencephalitis (i.e., brainstem encephalitis) is a pathology typically associated with Lm, and was first described in human by Eck in 1957.Citation45 Rhombencephalitis is characterized by progressive brainstem dysfunction. Clinical signs classically appear in two phases. In the first 4 to 10 d period, unspecific symptoms consisting in headache, malaise, nausea and vomiting can be present. In a subsequent period, asymmetrical cranial nerve deficits, cerebellar signs and hemiparesis or hemisensory defects, appear and can be concomitant to meningeal signs. Contrary to meningitis, rhombencephalitis occurs mostly in otherwise healthy people.Citation46,Citation47 Based on neuropathological analysis, it has been shown that these clinical signs correlate with inflammatory infiltrates predominantly present within nuclei, tracts and intraparenchymal parts of cranial nerves innervating the oropharynx (5th, 7th, 9th, 10th and 12th cranial nerves)Citation48 (see ). These sequential observations in human may indicate that cranial nerve is secondarily reached via the extension of the infection process from the rhombencephalon, rather than a route for the retrograde transport of Lm to the rhombencephalon.
Figure 2. Cranial nerves in human. A frame surrounds the nerves that emerge from the regions most frequently infected by Lm in the brainstem. Adapted from Patrick Lynch; Creative Commons Attribution 2.5 License 2006; www.patricklynch.net.
Neurolisteriosis in ruminants
Listeriosis is a common pathology in ruminants. Infection is supposed to occur mainly from absorption of contaminated silage and manifests as abortion and encephalitis. Diffuse meningitis and meningoencephalitis are very rare in ruminants, contrary to rhombencephalitis, which is the cardinal form of disease, and was first described as “circling disease” in sheep in New Zealand.Citation49 A recent neuropathological survey in Switzerland identified neurolisteriosis as the most prevalent CNS disease of small ruminants. The incidence of neurolisteriosis in this country ranges from 200 to 500 cases/million small ruminants, very significantly exceeding the incidence in human. Of note, and as in human, placental infection and CNS infection seem to be two distinct pathologies, as concomitant infection of placenta and CNS is classically not observed in ruminant.Citation20
As in human, rhombencephalitis seems to occur mainly in hosts without known predisposing factor. The incubation period is long, from 1 to 7 weeks.Citation50,Citation51 Clinical signs, such as “circling disease,” are linked to the CNS lesions, which are unilateral or bilateral brainstem and cranial nerve deficits.Citation52,Citation53 The cranial nerves mostly involved are the 5th cranial nerve (trigeminal), 7th (facialis) and 12th (hypoglossus), and to a lesser extent the 6th, 8th, 9th and 10th nerves.Citation54 The prominence of cranial nerve deficit in ruminant, and the characteristics of their feeding behavior make of the retrograde transport of Lm to the rhombencephalon an attractive hypothesis, which is supported by neuropathological findings (see below).
Pathophysiology of Neurolisteriosis
What can be learned from Lm interactions with other host barriers?
We have studied the molecular mechanisms underlying Lm ability to target and cross the intestinal and placental barriers in detail. We have shown that the Lm surface protein InlA mediates a species-specific interaction with its host receptor E-cadherin (Ecad) at the intestinal epithelium level, resulting in bacterial internalization in enterocytes and crossing of the intestinal barrier.Citation55 Early after contamination, Lm crosses this barrier via transcytosis in mucus producing cells and their neighbors, which express luminally accessible Ecad.Citation56 In contrast, InlB, another species-specific internalin of Lm that interacts with Met, is not involved in this process.Citation57,Citation58
We have also shown that Lm targeting and crossing of the maternal-fetal (MF) barrier is critically dependent upon InlA interaction with trophoblast Ecad, but this time only in conjunction with InlB interaction with Met.Citation57,Citation59 The strong requirement of InlA in Lm crossing of the intestinal and placental barriers has been independently shown epidemiologically, since Lm isolates expressing a functional InlA are very significantly associated with their clinical origin, and particularly their fetoplacental origin.Citation57,Citation60
These results demonstrate that the molecular mechanisms underlying Lm ability to target and cross the intestinal and MF barriers exhibit similarities but also critical differences. Remarkably, the BBB is also composed of Ecad- and Met-expressing cells, such as the microvascular endothelium and choroid plexus epithelium. Thus, the molecular mechanisms enabling Lm to cross the BBB may also implicate InlA and/or InlB, and thus also share similarities with Lm crossing of the intestinal and MF barriers.
Crossing of the blood-brain, the blood-CSF barriers, or both?
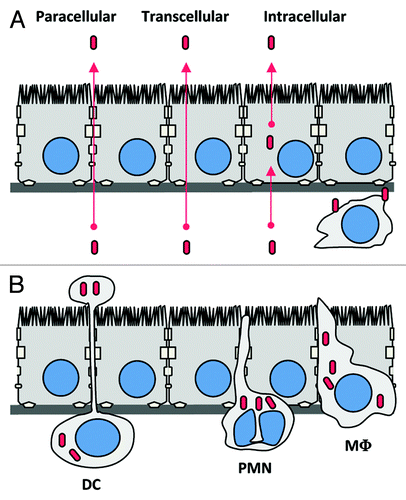
Lm-associated meningoencephalitis and rhombencephalitis appear to be two distinct forms of neurolisteriosis. Yet, it is not established whether the mechanisms underlying these two pathologies actually differ or share common steps. The hematogenous route of infection is favored for meningoencephalitis, whereas the neural retrograde route, described thereafter, has been proposed for Lm-associated rhombencephalitis in ruminants. Two major and non-exclusive ways for bacteria to cross the BBB are proposed, and described in : extracellular bacteria, either free in the blood and/or associated to cells, may recognize receptors at the surface of the barriers and cross them (); alternatively, and since Lm is a facultative intracellular bacterium, this bacterium may gain the CNS in infected cells, such as circulating leucocytes, which are known to be able to cross themselves the BBB ().Citation61
Figure 3. Potential mechanisms by which Lm could cross the blood-brain barrier. (A) Extracellular Lm, either free in the blood and/or associated to circulating cells, may recognize receptors at the surface of the barriers (as InlA, InlB or Vip) and cross them. (B) Trojan horse mechanism: Circulating leucocytes infected by Lm, such as monocytes, dendritic cells or polymorphonuclear cells, may cross the BBB hence targeting the bacteria in the CNS. DC, dendritic cell; PMN, polymorphonuclear leukocyte; M?, monocyte/macrophage.
Brain endothelial cells and choroid plexus epithelial cells form the blood-CNS barriers
Brain endothelial cells that constitute the blood-brain barrier differ in many ways from classical endothelial cells.Citation62 They are in contact with each other by tight junctions, made of occludin, claudins, junctional adhesion molecules (JAM) and cell-selective adhesion molecules that induce a high trans-endothelial resistance and prevent paracellular trafficking of macromolecules. In addition they express adherens junction proteins such as VE-cadherin and N-cadherin (). Rubin et al. have also proposed that brain endothelial cells express E-cadherin, although this result would need to be revisited with updated tools, as they predate the discovery of VE-cadherin.Citation63 Differentiation of these cells is allowed and maintained by an intimate interaction with astrocytes and their secreted products and neurons in what is referred as “neurovascular unit.”Citation64
Choroid plexus epithelial cells form the blood-CSF barrier. They are located in brain ventricles and produce CSF from a blood filtrate.Citation65 They are separated from the systemic circulation by fenestrated endothelial cells filled with blood and stromal cells (). As other epithelial cells, they express E-cadherin at their basolateral pole, as well as tight junctions and as such constitute an attractive site of entry for Lm in the CNS.
Direct interaction of extracellular bacteria with the blood-brain and blood-CSF barriers
As mentioned before, Lm has the capacity to interact via InlA and InlB with its cellular receptors E-cadherin and Met, respectively, to cross the intestinal and placental barriers. Since these two receptors are expressed at the surface of choroid plexus epithelial cells, and Met is also expressed at the brain endothelial level, similar mechanisms than at the intestinal and placental levels could occur at the blood-CSF and BBB, respectively.
It has been shown in vitro that Lm is able to invade human umbilical vein endothelial cells (HUVEC) in an InlB-dependent manner,Citation66 as well as human brain microvascular endothelial cells (HBMEC).Citation67 In these cells, bacteria can survive, replicate, move intracellularly by inducing actin tail formation and spread from cell to cell.Citation67,Citation68 However, widespread vasculitis is not observed in Lm infection and interaction with HBMEC may result in vivo in a rapid crossing of the barrier rather than local infection and replication in brain microvessel endothelium.
Another bacterial candidate is Vip, which can interact with its receptor the heat-shock protein Gp96 expressed at the brain microvessel surface.Citation69 It has been shown that Δvip strain is attenuated in the brain invasion upon intravenous inoculation, but also in other organs such as liver and spleen, which may indeed account for the attenuated CNS infection observed with the Δvip deletion mutant. Of note, Gp96 has been shown to be a potential receptor for another pathogen targeting the brain, Candida albicans,Citation70 while a related protein, Ec-Gp96, may also be involved in the targeting of HBMEC by E. coli K1, a prominent cause of neonatal meningitis.Citation71
Lm is able to induce, via the action of the pore forming toxin listeriolysin O (LLO) NFκB activation, both in cultured endothelial cells and in brain microvessels, and induce the expression of the surface adhesion molecules P- and E-selectin, ICAM-1 and V-CAM-1, as well as interleukin (IL) IL-6 and IL-8 and the chemoattractant MCP-1, which allow neutrophil and monocyte adhesion to the endothelial cells.Citation72-Citation74 These effects may modulate blood-brain barrier function and favor Lm access to the CNS.
Trojan horse model
The “Trojan horse” mechanism was first proposed for ovine CNS invasion by visna virus.Citation75 Lm is able to escape from the phagosome in a LLO-dependent manner and to move in the host cytosol in an ActA-dependent manner.Citation18 These properties enable Lm to survive and proliferate in phagocytic cells such as monocytes or dendritic cells both in vitro and in vivo.Citation76,Citation77 Infected phagocytic cells may translocate, as a Trojan horse, Lm into the CNS. In mice intravenously inoculated, Lm has been shown to infect the brain even after gentamicin treatment, which is thought to eradicate extracellular blood-borne bacteria.Citation19 This suggests that infected phagocytes may play a role in brain invasion. Further experiments showed that the main infected cells were Ly-6Chigh monocytes, which could be isolated from infected brains.Citation78 In another study of acute infection in mouse, it has been observed that bacteria invade bone marrow myelomonocytic cells expressing the phenotype CD31pos:Ly-6Cpos:CD11bpos:LY-6Glow.Citation79 Injection of Lm-infected bone marrow cells appeared to facilitate CNS invasion in comparison to free bacteria or infected splenocytes, indicating that these infected cells may disseminate to the CNS. Yet the observation of a concomitant higher bacterial load in blood probably rather indicates that they constitute a niche in which bacteria can survive and multiply. The mechanisms by which these cells target the CNS is not known, although this process has been shown as IFNγ-dependent but CCR2-independent.Citation80 In the infected host, the action of LLO on endothelial cells may also lead to ICAM-1 and P-selectin expression, which in turn would allow infected cells to adhere to brain microvessels or meningeal vessels and favor CNS invasion.Citation72,Citation81
When circulating in the brain vasculature, infected cells could translocate from the blood to the CNS. Direct neuronal infection is not a necessary step to induce brain damage upon infection. It has been observed that neurons are not easily directly infected, yet in vitro observations indicate that they could be infected indirectly by bacteria spreading from infected macrophages to neurons.Citation82 Bacteria may also spread from myeloid infected cells to endothelial cells and thus cross the BBB, although this has only been observed in vitro.Citation67 This phenotype has been shown to depend on both ActA (which mediates actin-based motility that result in intracytosolic movement and formation of bacteria contatining protrusions able to penetrate neighboring cells) and on phospholipase C PlcA and PlcB, (which are implicated in the lysis of the double membrane vacuole in the recipient cell).Citation67
Neural retrograde route
In ruminants and more generally in the process of rhombencephalitis, it has been proposed that infection via neural retrograde transport is the most likely to occur.Citation20 This hypothesis is based on the observation of the neuropathological pattern of natural disease. Oevermann et al. have reported the results of their detailed investigations on the neuropathology of more than 200 cases of Lm-associated encephalitis in cattle, sheep and goats from Switzerland.Citation54 They analyzed the anatomical distribution, severity and bacterial load and tried to define a posteriori the temporal evolution of the lesions. Their results suggest that Lm gains access to the brainstem via retrograde axonal migration along the trigeminal nerve branches and also along other cranial nerves. Moreover, Lm seems to spread further from the brainstem into rostral brain regions, likely by intracerebral axonal migration, as evidenced by histopathological analyses.
In an attempt to investigate in a murine model if Lm could induce rhombencephalitis via a retrograde neural route, bacteria were inoculated unilaterally into facial muscle, or peripheral parts of a cranial nerve. In this model, clinical and histological signs of mainly ipsilateral rhombencephalitis could be observed.Citation83 This suggests that rhombencephalitis could be caused by intra-axonal bacterial spread from peripheral sites to the central nervous system.
The way peripheral nerves become infected in the first place, prior to retrograde dissemination to the CNS remains unknown. It has been proposed that bacteria might enter through mucosal injury in the oropharyngeal cavity.Citation20 Interestingly, in human, case reports of rhombencephalitis have been described in patients after dental surgery,Citation84,Citation85 supporting this hypothesis. Clinical signs of rhombencephalitis have also been observed in orally inoculated mice after a scarification of the oral, nasal and labial mucosae.Citation50
Recently it has been proposed that E-cadherin could act as a receptor for Lm to gain access to the brainstem.Citation86 InlA receptor Ecad is indeed expressed in satellite cells and myelinating Schwann cells in cranial nerves and ganglia, and Lm can be observed in phagocytes, axons, Schwann cells, satellite cells and ganglionic neurons in ruminant biopsies. The authors suggest that the oral epithelium and Schwann cells expressing E-cadherin may provide a portal of entry for free bacteria, which may then invade the axonal compartment by cell-to-cell spread. To prove this hypothesis, an experimental model of rhombencephalitis in which the role of InlA could be tested is needed. We are currently working on this.
Schluter et al. demonstrated that once in the brain after intracerebral infection, InlA and InlB play no role in a mouse model, but PlcB, which is involved in vacuolar escape and cell-to-cell spread does.Citation87 Although we now know that InlA is inefficient in the mouse,Citation88 these results are in favor of an infection of neurons by a spread from cell to cell.
Which Bacterial Factor(s) May Be Implicated in Lm CNS Tropism?
Epidemiological analyses
We have shown that clinical samples express a full-length InlA far more frequently (96%) that strains recovered from food samples in which 35% of strains harbors a truncated InlA. This accounts for the role of InlA in crossing the intestinal barrier.Citation60 Full-length InlA is also statistically associated with maternofetal listeriosis compared with bacteremia, this again showing the role of InlA in invading the placenta and fetus.Citation57,Citation60 However, even if amount of full-length InlA strains in neurolisteriosis sample (98%) is higher than those from bacteremia (93%), this difference does not reach statistical significance. So far, involvement of InlA in inducing neurolisteriosis is unclear, even if it could play a role in rhombencephalitis (see above). Experiments in permissive animal models for InlA-E-cadherin interaction are under way and will allow knowing precisely the role of InlA in Lm CNS tropism in vivo.
In an attempt to find factors involved in neurolisteriosis, a multi-locus variable-number tandem-repeat analysis (MLVA) has been conducted on Lm isolates from ruminant rhombencephalitis and were compared with isolates from human patients, food and environment.Citation89 Allelic profile-based comparisons grouped Lm mainly into three clonal complexes. Isolates from human and ruminant brain samples were mainly located in clonal complex A, which contains all but one rhombencephalitis isolate from cattle. A larger study is now needed to compare isolates from CNS invasion to isolates from bacteremia in order to determine genes specifically involved in CNS invasion.
High-throughput methods
In order to determine genes that could be involved in CNS invasion in a mouse model of infection, Autret et al. induced brain infection combined with signature-tagged mutagenesis (STM),Citation90 and identified genes encoding cell wall components such as gtcA, which is involved in the glycosylation of teichoic acid, and ytgP, coding for a putative integral membrane protein possibly involved in polysaccharide biosynthesis. However, in a second STM experiment aimed at discovering genes involved in the survival in liver, the same gtcA and ytgP mutants were found to be attenuated,Citation91 suggesting a non-specialized role for these genes in the CNS invasion, which could be involved in the overall in vivo proliferation or survival of the bacterium.
New Animal Models are Needed to Study Neurolisteriosis
Even if advances in the pathophysiology of neurolisteriosis have been achieved from epidemiological analyses in human and ruminants, as well as from observations of naturally infected ruminant or experimentally infected mice, many questions remain about the different routes of CNS invasion. There is now a need for a small animal model of neurolisteriosis that would recapitulate the different steps and features of the CNS infection in human.
This model has to possess the following properties (1) to be, as in human, permissive for the known virulence factors InlA and InlB, (2) to develop the clinical hallmarks of human neurolisteriosis (meningitis and/or rhombencephalitis) and (3) to exhibit the neuroradiological and histopathological lesions observed in humans.
Mouse has been proven as an animal model of choice for studying immunity and the role of virulence factors of Lm after an intravenous injection, such as listeriolysin or actA. Different approaches have been performed to induce neurolisteriosis in mice. For all of them, one of the concerns is that mouse E-cadherin is not recognized by InlA, contrary to human E-cadherin, thereby questioning the relevance of results obtained in this model.Citation88,Citation92 A classical approach is to inject Lm in the tail vein. This method is adapted to study meningitis if bacteria cross the BBB from the bloodstream in an InlA-independent manner.Citation79 However, since Lm is a foodborne pathogen, this route of infection bypasses a critical stage of the infection, the crossing of the oro-intestinal barrier, which may affect the way by which bacteria access the bloodstream.
In order to study CNS infection in small animal models, rodents permissive to both InlA and InlB, such as gerbil and humanized mice could be used.Citation93 A first assay has been conducted in gerbils by Blanot et al.Citation94 However, bacteria where injected in the middle ear, a model highly relevant for CNS infections that stems from a contiguous infection of the upper airway apparatus (such as pneumococcal and hemophilus-associated meningitis) but which relevance is uncertain for a foodborne infection.
Of note, gerbil and humanized mice are permissive to InlA and InlB but, like any animal model, it cannot be excluded that other host-bacterial interactions that occur in human do not occur in one of these rodents. The reverse is true and interactions that occur in a given animal species may not occur in human. It is therefore important to study listeriosis in various animal species and to compare clinical, microbiological, neuroimaging data with what observed in human, in order to fully understand the pathophysiology of neurolisteriosis, and uncover its potential species-specific characteristics.
Conclusions
Neurolisteriosis is a major health and economical concern. Deciphering the mechanisms by which Lm gains access to the brain will help us not only understand a critical virulence attribute of Lm but also hopefully help improve the prognosis of this still very severe infection. Lm is a model to understand how pathogens cross host barriers, and studying neurolisteriosis and identifying novel bacterial and host factors implicated in the development of neurolisteriosis will likely be beneficial for a better understanding of the basic mechanisms underlying CNS infections.
| Abbreviations: | ||
| Lm | = | Listeria monocytogenes |
| CNS | = | central nervous system |
| CSF | = | cerebrospinal fluid |
| CT | = | computed tomography |
| BBB | = | blood-brain barrier |
Acknowledgments
Research in the microbes and host barriers group is financed by Institut Pasteur, Inserm, Fondation pour la Recherche Médicale, Ville de Paris, Fondation BNP-Paribas and the European Research Council. We thank the members of the Microbes and host barriers Group for their support, helpful discussion and advices.
References
- Sáez-Llorens X, McCracken GH Jr. Bacterial meningitis in children. Lancet 2003; 361:2139 - 48; http://dx.doi.org/10.1016/S0140-6736(03)13693-8; PMID: 12826449
- van de Beek D, de Gans J, Tunkel AR, Wijdicks EF. Community-acquired bacterial meningitis in adults. N Engl J Med 2006; 354:44 - 53; http://dx.doi.org/10.1056/NEJMra052116; PMID: 16394301
- Dumont J, Cotoni L. Bacille semblable au bacille du rouget du porc rencontré dans le liquide céphalo-rachidien d'un méningitique. Ann Inst Pasteur (Paris) 1921; 35:625 - 33
- Murray EGD, Webb RA, Swann MBR. A disease of rabbits characterised by a large mononuclear leucocytosis caused by hitherto undescribed bacillus Bacterium monocytogenes (n. sp.). J Pathol Bacteriol 1926; 29:407 - 39; http://dx.doi.org/10.1002/path.1700290409
- Pirie J. A new disease of veld rodents, “Tiger river disease”. Pub South Africa Inst Med Res 1927; 62:163 - 86
- Schlech WF 3rd, Lavigne PM, Bortolussi RA, Allen AC, Haldane EV, Wort AJ, et al. Epidemic listeriosis--evidence for transmission by food. N Engl J Med 1983; 308:203 - 6; http://dx.doi.org/10.1056/NEJM198301273080407; PMID: 6401354
- Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, et al. Listeria pathogenesis and molecular virulence determinants. Clin Microbiol Rev 2001; 14:584 - 640; http://dx.doi.org/10.1128/CMR.14.3.584-640.2001; PMID: 11432815
- Authority EFS. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2009. EFSA Journal 2011; 9:2090
- Mailles A, Lecuit M, Goulet V, Leclercq A, Stahl JP, National Study on Listeriosis Encephalitis Steering Committee. Listeria monocytogenes encephalitis in France. Med Mal Infect 2011; 41:594 - 601; http://dx.doi.org/10.1016/j.medmal.2011.07.009; PMID: 22036519
- Mailles A, Stahl JP, Steering Committee and Investigators Group. Infectious encephalitis in france in 2007: a national prospective study. Clin Infect Dis 2009; 49:1838 - 47; http://dx.doi.org/10.1086/648419; PMID: 19929384
- Tappero JW, Schuchat A, Deaver KA, Mascola L, Wenger JD. Reduction in the incidence of human listeriosis in the United States. Effectiveness of prevention efforts? The Listeriosis Study Group. JAMA 1995; 273:1118 - 22; http://dx.doi.org/10.1001/jama.1995.03520380054035; PMID: 7707600
- Voetsch AC, Angulo FJ, Jones TF, Moore MR, Nadon C, McCarthy P, et al, Centers for Disease Control and Prevention Emerging Infections Program Foodborne Diseases Active Surveillance Network Working Group. Reduction in the incidence of invasive listeriosis in foodborne diseases active surveillance network sites, 1996-2003. Clin Infect Dis 2007; 44:513 - 20; http://dx.doi.org/10.1086/511006; PMID: 17243053
- Goulet V, Hedberg C, Le Monnier A, de Valk H. Increasing incidence of listeriosis in France and other European countries. Emerg Infect Dis 2008; 14:734 - 40; http://dx.doi.org/10.3201/eid1405.071395; PMID: 18439354
- Gillespie IA, McLauchlin J, Little CL, Penman C, Mook P, Grant K, et al. Disease presentation in relation to infection foci for non-pregnancy-associated human listeriosis in England and Wales, 2001 to 2007. J Clin Microbiol 2009; 47:3301 - 7; http://dx.doi.org/10.1128/JCM.00969-09; PMID: 19675217
- Allerberger F, Wagner M. Listeriosis: a resurgent foodborne infection. Clin Microbiol Infect 2010; 16:16 - 23; http://dx.doi.org/10.1111/j.1469-0691.2009.03109.x; PMID: 20002687
- Huang SL, Chou YT, Hsieh YC, Huang YC, Lin TY, Chiu CH. Epidemiology and clinical characteristics of Listeria monocytogenes bacteremia in a Taiwanese medical center. J Microbiol Immunol Infect 2010; 43:485 - 90; http://dx.doi.org/10.1016/S1684-1182(10)60075-8; PMID: 21195975
- Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol 2004; 4:812 - 23; http://dx.doi.org/10.1038/nri1461; PMID: 15459672
- Hamon M, Bierne H, Cossart P. Listeria monocytogenes: a multifaceted model. Nat Rev Microbiol 2006; 4:423 - 34; http://dx.doi.org/10.1038/nrmicro1413; PMID: 16710323
- Drevets DA, Leenen PJ, Greenfield RA. Invasion of the central nervous system by intracellular bacteria. Clin Microbiol Rev 2004; 17:323 - 47; http://dx.doi.org/10.1128/CMR.17.2.323-347.2004; PMID: 15084504
- Oevermann A, Zurbriggen A, Vandevelde M. Rhombencephalitis Caused by Listeria monocytogenes in Humans and Ruminants: A Zoonosis on the Rise?. Interdiscip Perspect Infect Dis 2010; 2010:632513; http://dx.doi.org/10.1155/2010/632513; PMID: 20204066
- Guillet C, Join-Lambert O, Le Monnier A, Leclercq A, Mechaï F, Mamzer-Bruneel MF, et al. Human listeriosis caused by Listeria ivanovii. Emerg Infect Dis 2010; 16:136 - 8; http://dx.doi.org/10.3201/eid1601.091155; PMID: 20031061
- Eriksen L, Larsen HE, Christiansen T, Jensen MM, Eriksen E. An outbreak of meningo-encephalitis in fallow deer caused by Listeria monocytogenes. Vet Rec 1988; 122:274 - 6; http://dx.doi.org/10.1136/vr.122.12.274; PMID: 3131946
- Butt MT, Weldon A, Step D, De Lahunta A, Huxtable CR. Encephalitic listeriosis in two adult llamas (Lama glama): clinical presentations, lesions and immunofluorescence of Listeria monocytogenes in brainstem lesions. Cornell Vet 1991; 81:251 - 8; PMID: 1908758
- Avery RJ, Byrne JL. An Attempt To Determine The Incidence Of Listeria Monocytogenes In The Brain Of Mammals. Can J Comp Med Vet Sci 1959; 23:296 - 300; PMID: 17649178
- Raith K, Müntener T, Vandevelde M, Oevermann A. Encephalomyelitis resembling human and ruminant rhombencephalitis caused by Listeria monocytogenes in a feline leukemia virus-infected cat. J Vet Intern Med 2010; 24:983 - 5; http://dx.doi.org/10.1111/j.1939-1676.2010.0518.x; PMID: 20412434
- Langohr IM, Ramos-Vara JA, Wu CC, Froderman SF. Listeric meningoencephalomyelitis in a cougar (Felis concolor): characterization by histopathologic, immunohistochemical, and molecular methods. Vet Pathol 2006; 43:381 - 3; http://dx.doi.org/10.1354/vp.43-3-381; PMID: 16672590
- Aoyagi T, Sato Y, Matsuura S, Wada H. Listeriosis in a raccoon dog (Nyctereutes procyonoides) associated with canine distemper. J Vet Med Sci 2000; 62:639 - 41; http://dx.doi.org/10.1292/jvms.62.639; PMID: 10907692
- Clauss HE, Lorber B. Central nervous system infection with Listeria monocytogenes. Curr Infect Dis Rep 2008; 10:300 - 6; http://dx.doi.org/10.1007/s11908-008-0049-0; PMID: 18765103
- Mook-Kanamori BB, Geldhoff M, van der Poll T, van de Beek D. Pathogenesis and pathophysiology of pneumococcal meningitis. Clin Microbiol Rev 2011; 24:557 - 91; http://dx.doi.org/10.1128/CMR.00008-11; PMID: 21734248
- Rosenstein NE, Perkins BA, Stephens DS, Popovic T, Hughes JM. Meningococcal disease. N Engl J Med 2001; 344:1378 - 88; http://dx.doi.org/10.1056/NEJM200105033441807; PMID: 11333996
- Turk DC. The pathogenicity of Haemophilus influenzae. J Med Microbiol 1984; 18:1 - 16; http://dx.doi.org/10.1099/00222615-18-1-1; PMID: 6146721
- Thwaites GE, Tran TH. Tuberculous meningitis: many questions, too few answers. Lancet Neurol 2005; 4:160 - 70; PMID: 15721826
- Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol 2000; 30:1217 - 58; http://dx.doi.org/10.1016/S0020-7519(00)00124-7; PMID: 11113252
- Jantzen SU, Ferrea S, Langebner T, Gaebel W, Griese M, Arendt G, et al. Late-stage neurosyphilis presenting with severe neuropsychiatric deficits: diagnosis, therapy, and course of three patients. J Neurol 2012; 259:720 - 8; http://dx.doi.org/10.1007/s00415-011-6252-1; PMID: 21964751
- Rhoades RE, Tabor-Godwin JM, Tsueng G, Feuer R. Enterovirus infections of the central nervous system. Virology 2011; 411:288 - 305; http://dx.doi.org/10.1016/j.virol.2010.12.014; PMID: 21251690
- Brouwer MC, van de Beek D, Heckenberg SG, Spanjaard L, de Gans J. Community-acquired Listeria monocytogenes meningitis in adults. Clin Infect Dis 2006; 43:1233 - 8; http://dx.doi.org/10.1086/508462; PMID: 17051485
- Mylonakis E, Hohmann EL, Calderwood SB. Central nervous system infection with Listeria monocytogenes. 33 years’ experience at a general hospital and review of 776 episodes from the literature. Medicine (Baltimore) 1998; 77:313 - 36; http://dx.doi.org/10.1097/00005792-199809000-00002; PMID: 9772921
- Schuchat A, Robinson K, Wenger JD, Harrison LH, Farley M, Reingold AL, et al, Active Surveillance Team. Bacterial meningitis in the United States in 1995. N Engl J Med 1997; 337:970 - 6; http://dx.doi.org/10.1056/NEJM199710023371404; PMID: 9395430
- van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004; 351:1849 - 59; http://dx.doi.org/10.1056/NEJMoa040845; PMID: 15509818
- Merle-Melet M, Dossou-Gbete L, Maurer P, Meyer P, Lozniewski A, Kuntzburger O, et al. Is amoxicillin-cotrimoxazole the most appropriate antibiotic regimen for listeria meningoencephalitis? Review of 22 cases and the literature. J Infect 1996; 33:79 - 85; http://dx.doi.org/10.1016/S0163-4453(96)92929-1; PMID: 8889993
- Izbéki F, Nagy F, Szepes Z, Kiss I, Lonovics J, Molńr T. Severe Listeria meningoencephalitis in an infliximab-treated patient with Crohn’s disease. Inflamm Bowel Dis 2008; 14:429 - 31; http://dx.doi.org/10.1002/ibd.20286; PMID: 17973302
- Eckburg PB, Montoya JG, Vosti KL. Brain abscess due to Listeria monocytogenes: five cases and a review of the literature. Medicine (Baltimore) 2001; 80:223 - 35; http://dx.doi.org/10.1097/00005792-200107000-00001; PMID: 11470983
- Lorber B. Listeriosis. Clin Infect Dis 1997; 24:1 - 9, quiz 10-1; http://dx.doi.org/10.1093/clinids/24.1.1; PMID: 8994747
- Schlech WF 3rd. Foodborne listeriosis. Clin Infect Dis 2000; 31:770 - 5; http://dx.doi.org/10.1086/314008; PMID: 11017828
- Eck H. Encephalomyelitis listeriaca apostematosa. Schweiz Med Wochenschr 1957; 87:210 - 4; PMID: 13432650
- Antal EA, Dietrichs E, Løberg EM, Melby KK, Maehlen J. Brain stem encephalitis in listeriosis. Scand J Infect Dis 2005; 37:190 - 4; http://dx.doi.org/10.1080/00365540410020938; PMID: 15849051
- Armstrong RW, Fung PC. Brainstem encephalitis (rhombencephalitis) due to Listeria monocytogenes: case report and review. Clin Infect Dis 1993; 16:689 - 702; http://dx.doi.org/10.1093/clind/16.5.689; PMID: 8507761
- Antal EA, Løberg EM, Dietrichs E, Maehlen J. Neuropathological findings in 9 cases of listeria monocytogenes brain stem encephalitis. Brain Pathol 2005; 15:187 - 91; http://dx.doi.org/10.1111/j.1750-3639.2005.tb00519.x; PMID: 16196384
- Gill DA. “Circling” disease: a meningoencephalitis of sheep in New Zealand. Vet J 1933; 89:258 - 70
- Akiyama Y, Asahi O, Hosoda T. Studies on the mechanism of infection of the brain with Listeria monocytogenes. Am J Vet Res 1957; 18:147 - 57; PMID: 13394833
- Low JC, Donachie W. A review of Listeria monocytogenes and listeriosis. Vet J 1997; 153:9 - 29; http://dx.doi.org/10.1016/S1090-0233(97)80005-6; PMID: 9125353
- Braun U, Stehle C, Ehrensperger F. Clinical findings and treatment of listeriosis in 67 sheep and goats. Vet Rec 2002; 150:38 - 42; http://dx.doi.org/10.1136/vr.150.2.38; PMID: 11829065
- Schweizer G, Ehrensperger F, Torgerson PR, Braun U. Clinical findings and treatment of 94 cattle presumptively diagnosed with listeriosis. Vet Rec 2006; 158:588 - 92; http://dx.doi.org/10.1136/vr.158.17.588; PMID: 16648439
- Oevermann A, Di Palma S, Doherr MG, Abril C, Zurbriggen A, Vandevelde M. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol 2010; 20:378 - 90; http://dx.doi.org/10.1111/j.1750-3639.2009.00292.x; PMID: 19476464
- Lecuit M, Vandormael-Pournin S, Lefort J, Huerre M, Gounon P, Dupuy C, et al. A transgenic model for listeriosis: role of internalin in crossing the intestinal barrier. Science 2001; 292:1722 - 5; http://dx.doi.org/10.1126/science.1059852; PMID: 11387478
- Nikitas G, Deschamps C, Disson O, Niault T, Cossart P, Lecuit M. Transcytosis of Listeria monocytogenes across the intestinal barrier upon specific targeting of goblet cell accessible E-cadherin. J Exp Med 2011; 208:2263 - 77; http://dx.doi.org/10.1084/jem.20110560; PMID: 21967767
- Disson O, Grayo S, Huillet E, Nikitas G, Langa-Vives F, Dussurget O, et al. Conjugated action of two species-specific invasion proteins for fetoplacental listeriosis. Nature 2008; 455:1114 - 8; http://dx.doi.org/10.1038/nature07303; PMID: 18806773
- Khelef N, Lecuit M, Bierne H, Cossart P. Species specificity of the Listeria monocytogenes InlB protein. Cell Microbiol 2006; 8:457 - 70; http://dx.doi.org/10.1111/j.1462-5822.2005.00634.x; PMID: 16469057
- Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu MC, et al. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. Proc Natl Acad Sci U S A 2004; 101:6152 - 7; http://dx.doi.org/10.1073/pnas.0401434101; PMID: 15073336
- Jacquet C, Doumith M, Gordon JI, Martin PM, Cossart P, Lecuit M. A molecular marker for evaluating the pathogenic potential of foodborne Listeria monocytogenes. J Infect Dis 2004; 189:2094 - 100; http://dx.doi.org/10.1086/420853; PMID: 15143478
- Kim KS. Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol 2008; 6:625 - 34; http://dx.doi.org/10.1038/nrmicro1952; PMID: 18604221
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis 2010; 37:13 - 25; http://dx.doi.org/10.1016/j.nbd.2009.07.030; PMID: 19664713
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, et al. A cell culture model of the blood-brain barrier. J Cell Biol 1991; 115:1725 - 35; http://dx.doi.org/10.1083/jcb.115.6.1725; PMID: 1661734
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41 - 53; http://dx.doi.org/10.1038/nrn1824; PMID: 16371949
- Wolburg H, Paulus W. Choroid plexus: biology and pathology. Acta Neuropathol 2010; 119:75 - 88; http://dx.doi.org/10.1007/s00401-009-0627-8; PMID: 20033190
- Parida SK, Domann E, Rohde M, Müller S, Darji A, Hain T, et al. Internalin B is essential for adhesion and mediates the invasion of Listeria monocytogenes into human endothelial cells. Mol Microbiol 1998; 28:81 - 93; http://dx.doi.org/10.1046/j.1365-2958.1998.00776.x; PMID: 9593298
- Greiffenberg L, Goebel W, Kim KS, Weiglein I, Bubert A, Engelbrecht F, et al. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: InlB-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun 1998; 66:5260 - 7; PMID: 9784531
- Greiffenberg L, Goebel W, Kim KS, Daniels J, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: an electron microscopic study. Infect Immun 2000; 68:3275 - 9; http://dx.doi.org/10.1128/IAI.68.6.3275-3279.2000; PMID: 10816473
- Cabanes D, Sousa S, Cebrí A, Lecuit M, García-del Portillo F, Cossart P. Gp96 is a receptor for a novel Listeria monocytogenes virulence factor, Vip, a surface protein. EMBO J 2005; 24:2827 - 38; http://dx.doi.org/10.1038/sj.emboj.7600750; PMID: 16015374
- Liu Y, Mittal R, Solis NV, Prasadarao NV, Filler SG. Mechanisms of Candida albicans trafficking to the brain. PLoS Pathog 2011; 7:e1002305; http://dx.doi.org/10.1371/journal.ppat.1002305; PMID: 21998592
- Prasadarao NV, Srivastava PK, Rudrabhatla RS, Kim KS, Huang SH, Sukumaran SK. Cloning and expression of the Escherichia coli K1 outer membrane protein A receptor, a gp96 homologue. Infect Immun 2003; 71:1680 - 8; http://dx.doi.org/10.1128/IAI.71.4.1680-1688.2003; PMID: 12654781
- Kayal S, Lilienbaum A, Join-Lambert O, Li X, Israël A, Berche P. Listeriolysin O secreted by Listeria monocytogenes induces NF-kappaB signalling by activating the IkappaB kinase complex. Mol Microbiol 2002; 44:1407 - 19; http://dx.doi.org/10.1046/j.1365-2958.2002.02973.x; PMID: 12028384
- Krüll M, Nöst R, Hippenstiel S, Domann E, Chakraborty T, Suttorp N. Listeria monocytogenes potently induces up-regulation of endothelial adhesion molecules and neutrophil adhesion to cultured human endothelial cells. J Immunol 1997; 159:1970 - 6; PMID: 9257863
- Wilson SL, Drevets DA. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J Infect Dis 1998; 178:1658 - 66; http://dx.doi.org/10.1086/314490; PMID: 9815218
- Peluso R, Haase A, Stowring L, Edwards M, Ventura P. A Trojan Horse mechanism for the spread of visna virus in monocytes. Virology 1985; 147:231 - 6; http://dx.doi.org/10.1016/0042-6822(85)90246-6; PMID: 2998068
- Westcott MM, Henry CJ, Cook AS, Grant KW, Hiltbold EM. Differential susceptibility of bone marrow-derived dendritic cells and macrophages to productive infection with Listeria monocytogenes. Cell Microbiol 2007; 9:1397 - 411; http://dx.doi.org/10.1111/j.1462-5822.2006.00880.x; PMID: 17250592
- Pron B, Boumaila C, Jaubert F, Berche P, Milon G, Geissmann F, et al. Dendritic cells are early cellular targets of Listeria monocytogenes after intestinal delivery and are involved in bacterial spread in the host. Cell Microbiol 2001; 3:331 - 40; http://dx.doi.org/10.1046/j.1462-5822.2001.00120.x; PMID: 11298655
- Drevets DA, Dillon MJ, Schawang JS, Van Rooijen N, Ehrchen J, Sunderkötter C, et al. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J Immunol 2004; 172:4418 - 24; PMID: 15034057
- Join-Lambert OF, Ezine S, Le Monnier A, Jaubert F, Okabe M, Berche P, et al. Listeria monocytogenes-infected bone marrow myeloid cells promote bacterial invasion of the central nervous system. Cell Microbiol 2005; 7:167 - 80; http://dx.doi.org/10.1111/j.1462-5822.2004.00444.x; PMID: 15659061
- Drevets DA, Dillon MJ, Schawang JE, Stoner JA, Leenen PJ. IFN-gamma triggers CCR2-independent monocyte entry into the brain during systemic infection by virulent Listeria monocytogenes. Brain Behav Immun 2010; 24:919 - 29; http://dx.doi.org/10.1016/j.bbi.2010.02.011; PMID: 20211719
- López S, Prats N, Marco AJ. Expression of E-selectin, P-selectin, and intercellular adhesion molecule-1 during experimental murine listeriosis. Am J Pathol 1999; 155:1391 - 7; http://dx.doi.org/10.1016/S0002-9440(10)65241-8; PMID: 10514421
- Dramsi S, Lévi S, Triller A, Cossart P. Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study. Infect Immun 1998; 66:4461 - 8; PMID: 9712801
- Antal EA, Løberg EM, Bracht P, Melby KK, Maehlen J. Evidence for intraaxonal spread of Listeria monocytogenes from the periphery to the central nervous system. Brain Pathol 2001; 11:432 - 8; http://dx.doi.org/10.1111/j.1750-3639.2001.tb00411.x; PMID: 11556688
- Kennard C, Howard AJ, Scholtz C, Swash M. Infection of the brainstem by Listeria monocytogenes. J Neurol Neurosurg Psychiatry 1979; 42:931 - 3; http://dx.doi.org/10.1136/jnnp.42.10.931; PMID: 512667
- Medina-Flores R, Germanwala A, Molina JT, Meltzer CC, Wiley CA. October 2003: a 59-year-old woman with sudden onset of diplopia. Listerial rhombencephalitis. Brain Pathol 2004; 14:225 - 6; http://dx.doi.org/10.1111/j.1750-3639.2004.tb00100.x; PMID: 15193037
- Madarame H, Seuberlich T, Abril C, Zurbriggen A, Vandevelde M, Oevermann A. The distribution of E-cadherin expression in listeric rhombencephalitis of ruminants indicates its involvement in Listeria monocytogenes neuroinvasion. Neuropathol Appl Neurobiol 2011; 37:753 - 67; http://dx.doi.org/10.1111/j.1365-2990.2011.01183.x; PMID: 21486315
- Schlüter D, Domann E, Buck C, Hain T, Hof H, Chakraborty T, et al. Phosphatidylcholine-specific phospholipase C from Listeria monocytogenes is an important virulence factor in murine cerebral listeriosis. Infect Immun 1998; 66:5930 - 8; PMID: 9826375
- Lecuit M, Dramsi S, Gottardi C, Fedor-Chaiken M, Gumbiner B, Cossart P. A single amino acid in E-cadherin responsible for host specificity towards the human pathogen Listeria monocytogenes. EMBO J 1999; 18:3956 - 63; http://dx.doi.org/10.1093/emboj/18.14.3956; PMID: 10406800
- Balandyté L, Brodard I, Frey J, Oevermann A, Abril C. Ruminant rhombencephalitis-associated Listeria monocytogenes alleles linked to a multilocus variable-number tandem-repeat analysis complex. Appl Environ Microbiol 2011; 77:8325 - 35; http://dx.doi.org/10.1128/AEM.06507-11; PMID: 21984240
- Autret N, Dubail I, Trieu-Cuot P, Berche P, Charbit A. Identification of new genes involved in the virulence of Listeria monocytogenes by signature-tagged transposon mutagenesis. Infect Immun 2001; 69:2054 - 65; http://dx.doi.org/10.1128/IAI.69.4.2054-2065.2001; PMID: 11254558
- Autret N, Raynaud C, Dubail I, Berche P, Charbit A. Identification of the agr locus of Listeria monocytogenes: role in bacterial virulence. Infect Immun 2003; 71:4463 - 71; http://dx.doi.org/10.1128/IAI.71.8.4463-4471.2003; PMID: 12874326
- Lecuit M. Human listeriosis and animal models. Microbes Infect 2007; 9:1216 - 25; http://dx.doi.org/10.1016/j.micinf.2007.05.009; PMID: 17720601
- Disson O, Nikitas G, Grayo S, Dussurget O, Cossart P, Lecuit M. Modeling human listeriosis in natural and genetically engineered animals. Nat Protoc 2009; 4:799 - 810; http://dx.doi.org/10.1038/nprot.2009.66; PMID: 19444238
- Blanot S, Joly MM, Vilde F, Jaubert F, Clement O, Frija G, et al. A gerbil model for rhombencephalitis due to Listeria monocytogenes. Microb Pathog 1997; 23:39 - 48; http://dx.doi.org/10.1006/mpat.1997.0131; PMID: 9250779