Abstract
The ability of bacterial pathogens to sense their immediate environment plays a significant role on their capacity to survive and cause disease. Salmonella enterica serovar typhi (S. typhi) is an exclusively human pathogen that causes typhoid fever. In a recent study, we have shown that S. typhi senses and responds to host neuroendocrine stress hormones to release the toxin hemolysin E. Hormone-mediated hemolysis by S. typhi was inhibited by the β-blocker propranolol and was dependent on the presence of the CpxAR signal transduction system. Furthermore, we demonstrate that normal expression of the small RNA micA is necessary for the arbitration of the response to host neuroendocrine hormones. This leads to a significant decrease in the levels of the outer membrane protein OmpA and increased formation of membrane vesicles containing HlyE. The exploration of host pathogen interactions is of paramount importance in deciphering pathogen virulence and the discovery of novel treatments.
During the process of infection pathogenic organisms constantly monitor their environment and regulate virulence. Sensing and monitoring systems allow bacterial pathogens to survive in hostile environments. In addition, they provide valuable knowledge required for the bacteria to successfully navigate their way through the infectious cycle. Due to the intimate associations between hosts and pathogens, evolution has led to the development of a variety of mechanisms enabling pathogenic organisms to sense and spy on their hosts. Bacterial pathogens have developed systems for sensing host derived molecules. A class of signaling factors that have received considerable attention recently are the neuroendocrine stress hormones.Citation1,Citation2 The neuroendocrine stress hormone norepinephrine is abundant in the gut and epinephrine can be found in the bloodstream.Citation3 Interestingly, it has been shown that macrophages are also able to synthesize and respond to epinephrine and norepinephrine upon exposure to bacterial lipopolysaccharide.Citation4,Citation5
We have been exploring the ability of Salmonella to sense and respond to host neuroendocrine hormones.Citation6–Citation8 Our studies have for the first time documented a dual role for neuroendocrine hormone sensing in Salmonella. For example, in Salmonella enterica serovar typhimurium (S. typhimurium), epinephrine sensing may provide an environmental cue for the initiation of the Salmonella oxidative stress response in anticipation of imminent host-derived oxidative stress.Citation8 We showed that through iron transport, epinephrine affects the oxidative stress balance of the cell necessitating the stress regulator OxyR for normal growth.Citation8 On the other hand, the same epinephrine signaling may also serve in favor of the host defenses by lowering the pathogen's antimicrobial peptide resistance to polymyxin B.Citation8 Similarly, we have demonstrated that S. typhimurium sense epinephrine and downregulate resistance to the antimicrobial peptide LL-37 to the benefit of the host.Citation7 Thus, the balance of survival within the host versus eradication is dependent on a variety of constantly changing host-bacterial interactions.
In Escherichia coli O157:H7, epinephrine and norepinephrine signaling affects bacterial virulence and motility.Citation2 In E. coli O157:H7 the membrane sensor QseC acts as a bacterial adrenergic receptor to activate virulence genes in response to interkingdom cross-signaling.Citation9,Citation10 It was reported that S. typhimurium lacking qseC were defective in motility, epithelial cell invasion, and survival within macrophages. Consequently the qseC mutant was attenuated for systemic infection in 129 × 1/SvJ mice.Citation11 Several groups including ourselves have made alternative observations on the importance of qseBC. For example, transcriptional profiling of S. typhimurium qseB/qseC mutants revealed significant differences to the above observations including motility and responsiveness to host hormones or quorum sensing signal molecules.Citation12 Indeed the lack of qseBC in S. typhimurium did not significantly affect expression of the flagellar operon genes and, importantly, epinephrine increased S. typhimurium motility independently of qseBC.Citation12 The sensing of neuroendocrine stress hormones by S. typhimurium may not be exclusively by QseC. We have demonstrated that in S. typhimurium QseBC, and also QseEF, do not contribute to the adrenergic responses leading to increased sensitivity to the antimicrobial peptides polymyxin B and LL-37.Citation7,Citation8 Additionally, there is evidence that the QseC and QseE sensors are not required for norepinephrine-enhanced enteritis or intestinal colonization in calves.Citation13
Alternative strategies to investigate the importance of host produced neuroendocrine hormones in the virulence of S. typhimurium have been used by a number of groups. For example, transgenic mice defective in dopamine β-hydroxylase that are unable to produce epinephrine and norepinephrine have been used as hosts. When they were infected with S. typhimurium a modest positive effect on pathogenesis was evident suggesting that neuroendocrine stress hormones reduce the virulence of S. typhimurium or increase host resistance.Citation11 These transgenic mice also have defective Th1 T cell responses important for immunity to intracellular pathogens and this may have complicated the findings. In a model of porcine salmonellosis, the effect upon bacterial virulence of injecting the neurotoxin 6-hydroxydopamine to force the release of norepinephrine was found to be mild and transient leading to increased fecal shedding of S. typhimurium. Furthermore, the lack of qseC had no significant impact upon the outcome of the infection but reduced fecal shedding of qseC.Citation14
There are clear inconsistencies emerging in the data generated by different laboratories on the importance of QseC and QseE in bacterial adrenergic sensing in Salmonella. These discrepancies may reside in the varieties of artificial in vitro growth conditions used and the different host animals models employed. These problems have been further exacerbated by the use bacterial strains with differing genetic backgrounds. Importantly, direct binding and sensory activation assays by these hormones have not been documented for any of the proposed sensors in Salmonella. Therefore, any functional inferences proposed for the sensors are dependent on the in silico sequence similarities of the orthologs to qseC and qseE in E. coli O157:H7, and extrapolating phenotypes. The de facto constantly changing levels of neuroendocrine stress hormones also entails that the pathogen may have evolved sensors with varying levels of sensitivity. Moreover, one has to account for the pleiotropic phenotypic effects due to the ability of epinephrine and norepinephrine to modulate intracellular iron levels and hence growth.Citation1,Citation8 Such pleiotropic effects of qseC in E. coli O157:H7 and uropathogenic E. coli are described in a recent study which revealed perturbations in amino acid, nucleotide and carbon metabolism resulted in the downregulation of virulence factors.Citation15
The area of interkingdom communication research clearly merits further investigation and we turned our attentions to the exclusively human pathogen Salmonella enterica serovar typhi (S. typhi) to provide further detailed insights. This pathogen is predominantly systemic during infection and hence comes in contact with an array of host factors present in the blood including neuroendocrine hormones. Using a variety of physiological and molecular techniques, we have observed and subsequently elucidated the mechanistic basis of hormone-mediated hemolysis in S. typhi.Citation6 In both S. typhi and S. Paratyphi, hlyE (also known as clyA, sheA) encodes a hemolysin pore-forming toxin which may contribute to virulence and the development of systemic infections.Citation16,Citation17 Despite encoding the hemolysin HlyE, S. typhi is non-hemolytic on blood agar plates. Interestingly, clinical isolates fresh from the blood of typhoid patients may be transiently more hemolytic but lose hemolytic activity on passage (Dougan G, personal communication). Strikingly, exposure of the pathogen to the neuroendocrine stress hormones epinephrine and norepinephrine resulted in a zone of hemolysis on blood agar plates.Citation6 Furthermore the hemolysis was blockable in a liquid hemolysis assay using the β-adrenergic antagonist propranolol and was dependent on the presence of the hlyE gene.Citation6 The α-adrenergic blocker phentolamine had no effect on the response. In S. typhi HlyE is packaged into membrane vesicles (MVs), which are released in the external milieu to cause hemolysis.Citation18
To further elucidate the mechanistic basis of the hormone-induced hemolysis by S. typhi, we determined the effect of epinephrine and norepinephrine on the pathogen's proteome. We exposed S. typhi to 50 µM hormone as well as hormone/β-adrenergic blocker (propranolol) combinations and sampled at 30 min post-addition.Citation6 Levels of the mature form of the outer membrane protein A (OmpA) were significantly reduced (∼2.2-fold) upon exposure to epinephrine or norepinephrine. Importantly, overexpression of OmpA resulted in a significant loss of hormone mediated hemolysis by S. typhi, and addition of the β-adrenergic blocker propranolol prior to hormone treatment abolished the reduction in OmpA levels.Citation6
OmpA is a key regulator of bacterial osmotic homeostasis modulating the permeability and integrity of the outer membrane.Citation19 In S. typhi OmpA is crucial for maintaining envelope integrity and preventing hemolysis through MV secretion.Citation16 Indeed, we found that exposure of S. typhi to neuroendocrine hormones increased levels of MVs in supernatants independently of HlyE.Citation6
We speculated that by focusing on the regulatory cascade leading to production of OmpA in pathogens would further aid in the mechanistic dissection of the response to neuroendocrine hormones. The small RNA micA controls translation of the ompA transcript and hence levels of OmpA.Citation20 Neuroendocrine hormones significantly increased micA expression levels from a micA promoter-reporter as well as micA transcript levels.Citation6 The significant increase in the ompA translational repressor sRNA micA is in full accord with the observed reduction in OmpA levels. We have, therefore, proposed a micA-dependent signaling route for the hormone-mediated hemolytic cascade in S. typhi. In support of this hypothesis, chromosomal deletion of micA in S. typhi resulted in complete abolishment of hormone-mediated hemolysis.Citation6 Furthermore, overexpression of the micA transcript using arabinose induction fully restored hemolysis in the strain lacking micA.Citation6 Adding further support to this hypothesis, in cells exposed to neuroendocrine hormones we also observed a significant increase in levels of the small RNA chaperone Hfq, which is also known to mediate translational repression of OmpA.Citation6,Citation21
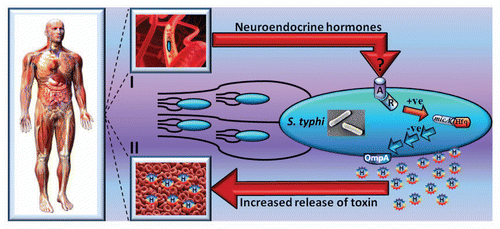
Having identified key players in the pathway leading to hormone-mediated hemolysis by S. typhi, we proceeded to investigate the nature of the bacterial adrenergic signal sensor. As mentioned earlier, in E. coli O157:H7 neuroendocrine hormones are sensed by the QseBC and QseEF two component systems.Citation10 Hormone-mediated hemolysis was indistinguishable from the parent strain in qseBC and qseEF mutants of S. typhi (data not shown). An in silico search for orthologs of qseBC in S. typhi, identified the cpxA sensor kinase which shares ∼29% amino acid sequence identity with the QseC sensor kinase. The cpxAR two component signal transduction system elicits the pathogens response to a diverse range of envelope stresses and is important for the pathogenicity of Salmonella in vivo.Citation22 Neuroendocrine stress hormones failed to induce hemolysis in a S. typhi strain lacking cpxAR.Citation6 Most importantly, complementation of the cpx system in trans from a single copy plasmid fully restored the hemolytic phenotype upon exposure to NE hormone.Citation6 Therefore in S. typhi, neuroendocrine hormone-mediated hemolysis is clearly independent of the known E. coli O157:H7 adrenergic sensory system QseBC and is mediated via a pathway requiring the CpxAR two component system. Mechanistically, it is possible that CpxAR may either directly or indirectly participate in the sensing of neuroendocrine hormones resulting in increased levels of Hfq and the sRNA micA. Consequently, micA and possibly Hfq suppress ompA translation leading to reduced OmpA and increased HlyE release within MVs.Citation6 The proposed interaction between S. typhi and its human host is depicted diagrammatically in .
The role of neuroendocrine stress hormones in S. typhi pathogenesis has so far been hindered due to the strong host-specificity barrier that averts S. typhi replication in species other than human. The recent arrival of new “humanized” mice models using immunodeficient mice engrafted with human hematopoietic stem and progenitor cells,Citation23,Citation24 although presenting an artificial human environment, will provide a valuable model system for further studies. It would be interesting to assess the single or combined roles of two component systems such as QseBC, QseEF, CpxAR and BasSR on the ability of S. typhi to survive and colonize such model systems.
Collectively, these studies suggest the existence of multiple adrenergic sensors in bacterial pathogens. The incessant evolution of pathogen virulence may have resulted in the recruitment and specialization of different sensors for the detection of specific host signaling cues. Efficient orchestration of the S. typhi response to host signals may require multiple alternative sensing pathways. The ability of S. typhi to detect neuroendocrine stress hormones like epinephrine and norepinephrine by an alternative sensor to QseBC/QseEF may reflect differences in the pathophysiology of the disease when compared to E. coli O157:H7. The unraveling of novel host-pathogen communication modules could provide unique targets for the next generation of antimicrobials to control infection.
Figures and Tables
Figure 1 Diagrammatical illustration of the interaction between S. typhi and host neuroendocrine stress hormones in the human systemic circulation leading to hemolysis of red blood cells. Hormones are sensed by S. typhi in the circulation (I) via a pathway requiring the CpxAR bacterial two component sensory transduction system (A and R respectively). Molecular signaling increases levels of the small RNA chaperone protein Hfq and the small RNA micA, thus, lowering the concentration of the outer membrane protein OmpA. Consequently, there is augmented shedding of the toxin hemolysin E (H) in membrane vesicles and hemolysis of red blood cells (II). Some of the images used in this composite figure are freeware courtesy of www.sciencephoto.com and www.oxygen-review.com.
Acknowledgments
The authors were supported by research grants from the Medical Research Council, UK. We also like to thank an anonymous reviewer for valuable comments, and Tracey Davey and Vivian Thompson for help with obtaining the SEM image of S. typhi in (Electron Microscopy Research Services, Newcastle University).
Addendum to:
References
- Freestone PPE, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol 2008; 16:55 - 64
- Pacheco AR, Sperandio V. Inter-kingdom signaling: chemical language between bacteria and host. Curr Opin Microbiol 2009; 12:192 - 198
- Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Sys 2000; 81:87 - 96
- Flierl MA, Rittirsch D, Nadeau BA, Chen AJ, Sarma JV, Zetoune FS, et al. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature 2007; 449:721 - 725
- Flierl MA, Rittirsch D, Nadeau BA, Sarma JV, Day DE, Lentsch AB, et al. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS ONE 2009; 4:4414
- Karavolos MH, Bulmer DM, Spencer H, Rampioni G, Schmalen I, Baker S, et al. Salmonella Typhi sense host neuroendocrine stress hormones and release the toxin haemolysin E. EMBO Rep 2011; 12:252 - 258
- Spencer H, Karavolos MH, Bulmer DM, Aldridge P, Chhabra SR, Winzer K, et al. Genome-wide transposon mutagenesis identifies a role for host neuroendocrine stress hormones in regulating the expression of virulence genes in S. typhimurium. J Bacteriol 2010; 192:714 - 724
- Karavolos MH, Spencer H, Bulmer DM, Thompson A, Winzer K, Williams P, et al. Adrenaline modulates the global transcriptional profile of Salmonella revealing a role in the antimicrobial peptide and oxidative stress resistance responses. BMC Genom 2008; 9:458
- Clarke MB, Hughes DT, Zhu C, Boedeker EC, Sperandio V. The QseC sensor kinase: A bacterial adrenergic receptor. Proc Natl Acad Sci USA 2006; 103:10420 - 10425
- Rasko DA, Moreira CG, Li DR, Reading NC, Ritchie JM, Waldor MK, et al. Targeting QseC signaling and virulence for antibiotic development. Science 2008; 321:1078 - 1080
- Moreira CG, Weinshenker D, Sperandio V. QseC mediates Salmonella enterica Serovar Typhimurium virulence in vitro and in vivo. Infect Immun 2010; 78:914 - 926
- Merighi M, Septer A, Carroll-Portillo A, Bhatiya A, Porwollik S, McClelland M, et al. Genome-wide analysis of the PreA/PreB (QseB/QseC) regulon of Salmonella enterica serovar Typhimurium. BMC Microbiol 2009; 9:42
- Pullinger GD, Carnell SC, Sharaff FF, van Diemen PM, Dziva F, Morgan E, et al. Norepinephrine augments Salmonella enterica-induced enteritis in a manner associated with increased net replication but independent of the putative adrenergic sensor kinases QseC and QseE. Infect Immun 2010; 78:372 - 380
- Pullinger GD, van Diemen PM, Carnell SC, Davies H, Lyte M, Stevens MP. 6-hydroxydopamine-mediated release of norepinephrine increases faecal excretion of Salmonella enterica serovar Typhimurium in pigs. Vet Res 2010; 41:68
- Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Mol Microbiol 2011; 80:1516 - 1529
- Fuentes JA, Villagra N, Castillo-Ruiz M, Mora GC. The Salmonella Typhi hlyE gene plays a role in invasion of cultured epithelial cells and its functional transfer to S. typhimurium promotes deep organ infection in mice. Res Microbiol 2008; 159:279 - 287
- von Rhein C, Bauer S, López Sanjurjo EJ, Benz R, Goebel W, Ludwig A. ClyA cytolysin from Salmonella: Distribution within the genus, regulation of expression by SlyA and pore-forming characteristics. Intl J Med Microbiol 2009; 299:21 - 35
- Wai SN, Lindmark B, Söderblom T, Takade A, Westermark M, Oscarsson J, et al. Vesicle-mediated export and assembly of pore-forming oligomers of the enterobacterial ClyA cytotoxin. Cell 2003; 115:25 - 35
- Wang Y. The function of OmpA in Escherichia coli. Biochem Biophys Res Comm 2002; 292:396 - 401
- Udekwu KI, Darfeuille F, Vogel J, Reimegard J, Holmqvist E, Wagner EG. Hfq-dependent regulation of OmpA synthesis is mediated by an antisense RNA. Genes Dev 2005; 19:2355 - 2366
- Vytvytska O, Moll I, Kaberdin VR, von Gabain A, Bläsi U. Hfq (HF1) stimulates ompA mRNA decay by interfering with ribosome binding. Genes Dev 2000; 14:1109 - 1118
- Humphreys S, Rowley G, Stevenson A, Anjum MF, Woodward MJ, Gilbert S, et al. Role of the two-component regulator CpxAR in the virulence of Salmonella enterica serotype Typhimurium. Infect Immun 2004; 72:4654 - 4661
- Firoz Mian M, Pek EA, Chenoweth MJ, Ashkar AA. Humanized mice are susceptible to Salmonella typhi infection. Cell Mol Immunol 2011; 8:83 - 87
- Song J, Willinger T, Rongvaux A, Eynon EE, Stevens S, Manz MG, et al. A mouse model for the human pathogen Salmonella Typhi. Cell Host Microbe 2010; 8:369 - 376