Abstract
Nine individuals with ulcerative colitis or Crohn disease grew up or lived in Plains, Montana, a 1,200-person community adjacent to the Clark Fork River near herds of free ranging Rocky Mountain bighorn sheep. This inflammatory bowel disease outbreak is similar to others that have occurred along rivers contaminated by animal feces.
Introduction
Over a decade ago, Fredricks and RelmanCitation1 proposed that the majority of so called “autoimmune” diseases such as ulcerative colitis and Crohn disease (the two major forms of inflammatory bowel disease) are actually infectious in etiology. They based their proposal in part on the publication in the medical literature of time-space “clusters,” which are groups of individuals, whether related or not, who live near one another and develop a disease within a relatively limited time period.
Most published clusters are actually outbreaks, since “outbreak” refers to aggregations of disease that are greater than expected, while “clusters” are aggregations that are not necessarily greater than expected.Citation2 In Mankato, Minnesota,Citation3 seven of 285 contacted members of the Mankato West High School class of 1981 have Crohn disease, an “extraordinary” prevalence rate of 2,400/100,000, in contrast to nearby Olmsted County, Minnesota’s prevalence rate of 174/100,000.Citation4 In Forest, Virginia, three of an estimated 60 individuals with Crohn disease or ulcerative colitis from a community of 8,006 developed Crohn disease within a six month period, an incidence rate almost 50 times the expected rate.Citation2 In Northport, Washington a reportedCitation5 54 individuals from a community of 330 have ulcerative colitis or Crohn disease, a combined prevalence rate of over 16,300/100,000. These three American inflammatory bowel disease (IBD) outbreaks, as well as particularly high rates of Crohn disease in Spokane, Washington in the US,Citation6 Cardiff in Wales,Citation7 Winnipeg, Manitoba in CanadaCitation8,Citation9 and Canterbury in New ZealandCitation9,Citation10 have occurred in communities located near rivers, lakes and creeks whose surface waters are contaminated by dairy cattle and/or domesticated sheep feces.
Mycobacterium avium subspecies paratuberculosis (MAP), the cause of a chronic gastrointestinal disease in dairy cattle, domestic sheep and other animals called Johne (“Yo-knee”) disease,Citation11 is excreted in an infected animal’s feces and so is consistent with the putative microorganism linked to previously reported IBD outbreaks. MAP is the long-suspected cause of Crohn diseaseCitation12 and has recently been proposed as the possible etiologic agent of ulcerative colitis as well.Citation13
While previous IBD outbreaks have been linked to dairy cattle and/or domestic sheep, the following is the first report of an IBD outbreak associated with a wild animal, the free ranging Rocky Mountain bighorn sheep.
Findings and Discussion
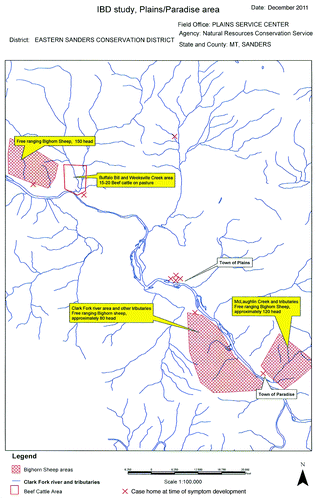
The seven Crohn disease and two ulcerative colitis cases were either born in Plains or moved there before developing symptoms of IBD. Their average age at symptom onset was 22 y, with a range from 9 to 53 y. The first case developed symptoms in 1995, the last in 2008. Some of the cases lived in multiple homes in Plains; indicates the home the case was living in at the time of symptom onset.
Figure 1. Map of the 1995–2008 Plains, Montana IBD outbreak. Note that all of the case homes are adjacent to the Clark Fork River or along one of its tributaries.
As with previous outbreaks, the greatly increased prevalence and incidence rates support the idea of an infectious etiology. The point prevalence rate of IBD in Plains is 9/1,200, or 750/100,000, almost double the normal prevalence rate in high incidence countries.Citation4 Two of the Crohn cases developed symptoms within a three month period at most, for an incidence rate of two Crohn disease cases/1,200/3 mo, more than 75 times the “expected” rate of 7.9 cases/100,000/year.Citation4
The fact that this and previously reported IBD outbreaks as well as unusually high rates of Crohn disease have occurred near rivers, lakes and other bodies of water contaminated by a probably MAP-infected animal’s feces suggests that a possible route of transmission of MAP from an infected animal to humans is indirect fecal-oral transmission via the inhalation of MAP-contaminated aerosolized water droplets. The inhalation of MAP-contaminated water droplets as a possible route of transmission of MAP from an infected animal’s feces to humans was first proposed by Hermon-Taylor and colleagues in 2000Citation14 to explain the ongoing outbreakCitation15 of Crohn disease in Cardiff, and in 2005 his group identified MAP in the water of the Taff River that runs through that city.Citation7 In Forest, Virginia, both the creeks that are the source of MAP-contaminated aerosolized water droplets, and the dairy cows whose manure is contaminating those creeks, are literally in several of the Forest cases’ backyards.Citation7 In Northport, Washington, 400–500 small dairies were located along the Sheep Creek that runs through Northport in the 1940s, 1950s and 1960s, and the dairy barns were deliberately constructed above the creek so manure was deposited directly into it. While direct fecal-oral, rather than inhalation, is a major route of transmission of MAP from one dairy animal to another, the inhalation of MAP-contaminated aerosolized water droplets has been proposedCitation16 and is now an experimentally documentedCitation17 source of bovine intestinal infection.
The aerosolization of MAP-contaminated water into small droplets capable of being inhaled and reaching human alveoli occurs in two steps, whether in outdoor or indoor environments. Indoors, MAP’s resistance to chlorination,Citation18 production of and adherence to biofilms,Citation19 and adherence to both plastic and metallic water pipesCitation20,Citation21 causes it to be concentrated in potable or drinking water.Citation21,Citation22 Outdoors, MAP’s extremely hydrophobic cell wall causes it to float to and be concentrated at the surfaceCitation23 of a natural body of water such as a river, lake or creek.Citation24
Step two of the aerosolization process is the actual aerosolization of water into small jetCitation25 and filmCitation26 drops that further concentrate MAP organisms. In indoor environments, the exit of water from bathroom faucetsCitation27 and showerheadsCitation28 and playing in baths filled with water are some of the mechanisms of water droplet aerosolization. The historically high rates of IBD in urban as compared with rural environmentsCitation29 as well as the reported association between Crohn disease and domestic hygieneCitation30 might not be due to the lack of exposure to a variety of enteric organisms but instead to specific exposure to highly concentrated doses of MAP spraying out of showerheadsCitation31 and bathtub faucets.Citation27
In outdoor environments,Citation25,Citation26 precipitation, the movement of water against the creek, stream or riverbank, and playing, swimming or splashing about are some of the means by which air bubbles are created within natural bodies of water. The air bubbles then “scavenge”Citation32 MAP organisms in the bulk water and those concentrated in the surface microlayer.Citation23 The jet and film drops created by the air bubbles bursting from the water’s surface again further concentrate MAP organisms.Citation25,Citation26 MAP is a member of the MAI (Mycobacterium avium intracellulare) complex of microorganisms, which are preferentially aerosolized from natural bodies of water in very high concentrations.Citation33,Citation34
Symptom onset for four of the nine cases occurred in the summer months of June, July or August, and for another four cases in the fall months of October or November, similar to the pattern of symptom onset of acute gastrointestinal illnesses following periods of both high precipitation (spring) and low (summer) in areas with heavy livestock and thus manure density.Citation35 Precipitation is one of the mechanisms for air bubble and water droplet production and MAP organism concentration as described in step two of the aerosolization process, perhaps accounting for the increase in cases in the summer months following northern hemisphere spring rainfall. Evaporation of water from the Clark Fork River in the hot summer months further concentrates MAP in the already concentrated surface microlayer of the river as described in step one of the aerosolization process, perhaps accounting for the increase in cases in the fall months.
Dairy cattle were the first animal species described with gastrointestinal disease caused by MAP infection, and are the probable source of MAP in the Forest, VirginiaCitation2 and Northport, WashingtonCitation5 American IBD outbreaks. There are, however, no dairy cattle within a 70-mile radius of Plains. MAP is known to cause gastrointestinal disease in a wide variety of animal species other than dairy cattle.Citation11 In this outbreak, the two possible sources of MAP are a single small beef cattle herd, and three large herds of free ranging Rocky Mountain bighorn sheep (). Given the small size of the nearby beef herd (less than 20 head) and the low prevalence of MAP infection in beef cattle,Citation36 the bighorn sheep are the likely source of MAP contaminating the Clark Fork River. Such sheep are known to be infected with MAP, to develop gastrointestinal disease and to shed MAP in their feces.Citation37-Citation40
Although MAP can be identified in human and Bighorn sheep blood,Citation40,Citation41 tissueCitation37,Citation38,Citation42 and fecesCitation39,Citation43 and in droplets aerosolized from natural bodies of water,Citation33 the author suffers from catastrophic and irreversible complications of Crohn diseaseCitation44 and is unable to further evaluate this outbreak. Attempts by the families in this and the Forest, Virginia IBD outbreaks to elicit evaluation and investigation by state public health authorities and the United States’ Center for Disease Control and Prevention have been met with either silence or repeated and pointed refusal. In private correspondence, MAP researchers have expressed their frustration with the refusal of IBD-related organizations and government agencies to fund any research related to MAP. One prominent researcher has alluded to this difficulty, having to resort to “donations from people suffering from Crohn disease, their families and friends.”Citation9 The publication of yet another American IBD outbreak will hopefully begin to encourage a reappraisal of public health and research priorities.
Acknowledgments
The author is indebted to the cases and their families who brought their outbreak to her attention and gave written permission to be included in this report. She is also most grateful for the assistance of an employee of the United States Department of Agriculture’s office in Plains, Montana for the preparation of and information contained in Figure 1, and, as always, Dr Dodie Ruzicki and the librarians of Providence Sacred Heart Medical Center and Children’s Hospital’s Health Sciences Library in Spokane, WA for the references. The author declares that no competing interests exist. The author received no funding for this case report.
References
- Fredricks DN, Relman DA. Infectious agents and the etiology of chronic idiopathic diseases. Curr Clin Top Infect Dis 1998; 18:180 - 200; PMID: 9779355
- Pierce ES, Borowitz SM, Naser SA. The Broad Street pump revisited: dairy farms and an ongoing outbreak of inflammatory bowel disease in Forest, Virginia. Gut Pathog 2011; 3:20; http://dx.doi.org/10.1186/1757-4749-3-20; PMID: 22196128
- Van Kruiningen HJ, Freda BJ. A clustering of Crohn’s disease in Mankato, Minnesota. Inflamm Bowel Dis 2001; 7:27 - 33; http://dx.doi.org/10.1097/00054725-200102000-00004; PMID: 11233657
- Loftus CG, Loftus EV Jr., Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ 3rd, et al. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota, 1940-2000. Inflamm Bowel Dis 2007; 13:254 - 61; http://dx.doi.org/10.1002/ibd.20029; PMID: 17206702
- Kramer B. Study tracks reasons for high rate of illness near Northport. Spokesman Review. Spokane, Washington, 2011 Feb 6. http://www.spokesman.com/stories/2011/feb/06/study-tracks-reasons-behind-high-rate-of-illness/
- Nunes GC, Ahlquist RE Jr. Increasing incidence of Crohn’s disease. Am J Surg 1983; 145:578 - 81; http://dx.doi.org/10.1016/0002-9610(83)90095-8; PMID: 6846693
- Pickup RW, Rhodes G, Arnott S, Sidi-Boumedine K, Bull TJ, Weightman A, et al. Mycobacterium avium subsp. paratuberculosis in the catchment area and water of the River Taff in South Wales, United Kingdom, and its potential relationship to clustering of Crohn’s disease cases in the city of Cardiff. Appl Environ Microbiol 2005; 71:2130 - 9; http://dx.doi.org/10.1128/AEM.71.4.2130-2139.2005; PMID: 15812047
- Green C, Elliott L, Beaudoin C, Bernstein CN. A population-based ecologic study of inflammatory bowel disease: searching for etiologic clues. Am J Epidemiol 2006; 164:615 - 23, discussion 624-8; http://dx.doi.org/10.1093/aje/kwj260; PMID: 16920784
- Hermon-Taylor J. Mycobacterium avium subspecies paratuberculosis, Crohn’s disease and the Doomsday scenario. Gut Pathog 2009; 1:15; http://dx.doi.org/10.1186/1757-4749-1-15; PMID: 19602288
- Gearry RB, Richardson A, Frampton CM, Collett JA, Burt MJ, Chapman BA, et al. High incidence of Crohn’s disease in Canterbury, New Zealand: results of an epidemiologic study. Inflamm Bowel Dis 2006; 12:936 - 43; http://dx.doi.org/10.1097/01.mib.0000231572.88806.b9; PMID: 17012964
- Clarke CJ. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol 1997; 116:217 - 61; http://dx.doi.org/10.1016/S0021-9975(97)80001-1; PMID: 9147244
- Uzoigwe JC, Khaitsa ML, Gibbs PS. Epidemiological evidence for Mycobacterium avium subspecies paratuberculosis as a cause of Crohn’s disease. Epidemiol Infect 2007; 135:1057 - 68; http://dx.doi.org/10.1017/S0950268807008448; PMID: 17445316
- Pierce ES. Ulcerative colitis and Crohn’s disease: is Mycobacterium avium subspecies paratuberculosis the common villain?. Gut Pathog 2010; 2:21; http://dx.doi.org/10.1186/1757-4749-2-21; PMID: 21167058
- Hermon-Taylor J, Bull TJ, Sheridan JM, Cheng J, Stellakis ML, Sumar N. Causation of Crohn’s disease by Mycobacterium avium subspecies paratuberculosis. Can J Gastroenterol 2000; 14:521 - 39; PMID: 10888733
- Rose JD, Roberts GM, Williams G, Mayberry JF, Rhodes J. Cardiff Crohn’s disease jubilee: the incidence over 50 years. Gut 1988; 29:346 - 51; http://dx.doi.org/10.1136/gut.29.3.346; PMID: 3356366
- Corner LA, Pfeiffer DU, Abbott KA. The respiratory tract as a hypothetical route of infection of cattle with Mycobacterium avium subspecies paratuberculosis. Aust Vet J 2004; 82:170 - 3; http://dx.doi.org/10.1111/j.1751-0813.2004.tb12652.x; PMID: 15088986
- Eisenberg SW, Koets AP, Nielen M, Heederik D, Mortier R, De Buck J, et al. Intestinal infection following aerosol challenge of calves with Mycobacterium avium subspecies paratuberculosis. Vet Res 2011; 42:117; http://dx.doi.org/10.1186/1297-9716-42-117; PMID: 22136728
- Falkinham JO 3rd. Factors influencing the chlorine susceptibility of Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum. Appl Environ Microbiol 2003; 69:5685 - 9; http://dx.doi.org/10.1128/AEM.69.9.5685-5689.2003; PMID: 12957962
- Wu CW, Schmoller SK, Bannantine JP, Eckstein TM, Inamine JM, Livesey M, et al. A novel cell wall lipopeptide is important for biofilm formation and pathogenicity of Mycobacterium avium subspecies paratuberculosis. Microb Pathog 2009; 46:222 - 30; http://dx.doi.org/10.1016/j.micpath.2009.01.010; PMID: 19490829
- Falkinham JO 3rd, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol 2001; 67:1225 - 31; http://dx.doi.org/10.1128/AEM.67.3.1225-1231.2001; PMID: 11229914
- Whiley H, Keegan A, Giglio S, Bentham R. Mycobacterium avium complex - the role of potable water in disease transmission. J Appl Microbiol 2012; 113:223 - 32; http://dx.doi.org/10.1111/j.1365-2672.2012.05298.x; PMID: 22471411
- Beumer A, King D, Donohue M, Mistry J, Covert T, Pfaller S. Detection of Mycobacterium avium subsp. paratuberculosis in drinking water and biofilms by quantitative PCR. Appl Environ Microbiol 2010; 76:7367 - 70; http://dx.doi.org/10.1128/AEM.00730-10; PMID: 20817803
- Hatcher RF, Parker BC. Microbiological and chemical enrichment of freshwater-surface microlayers relative to the bulk-subsurface water. Can J Microbiol 1974; 20:1051 - 7; http://dx.doi.org/10.1139/m74-162; PMID: 4837580
- Falkinham JO 3rd. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol 2009; 107:356 - 67; http://dx.doi.org/10.1111/j.1365-2672.2009.04161.x; PMID: 19228258
- Blanchard DC, Syzdek L. Mechanism for the water-to-air transfer and concentration of bacteria. Science 1970; 170:626 - 8; http://dx.doi.org/10.1126/science.170.3958.626; PMID: 4919182
- Blanchard DC, Syzdek LD. Water-to-Air Transfer and Enrichment of Bacteria in Drops from Bursting Bubbles. Appl Environ Microbiol 1982; 43:1001 - 5; PMID: 16346001
- Nishiuchi Y, Tamura A, Kitada S, Taguri T, Matsumoto S, Tateishi Y, et al. Mycobacterium avium complex organisms predominantly colonize in the bathtub inlets of patients’ bathrooms. Jpn J Infect Dis 2009; 62:182 - 6; PMID: 19468176
- du Moulin GC, Stottmeier KD, Pelletier PA, Tsang AY, Hedley-Whyte J. Concentration of Mycobacterium avium by hospital hot water systems. JAMA 1988; 260:1599 - 601; http://dx.doi.org/10.1001/jama.1988.03410110107037; PMID: 3411741
- Mendeloff AI. The epidemiology of inflammatory bowel disease. Clin Gastroenterol 1980; 9:259 - 70; PMID: 7389170
- Gent AE, Hellier MD, Grace RH, Swarbrick ET, Coggon D. Inflammatory bowel disease and domestic hygiene in infancy. Lancet 1994; 343:766 - 7; http://dx.doi.org/10.1016/S0140-6736(94)91841-4; PMID: 7907734
- Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci U S A 2009; 106:16393 - 9; http://dx.doi.org/10.1073/pnas.0908446106; PMID: 19805310
- Blanchard DC, Syzdek LD. Bubble scavenging of bacteria in freshwater quickly produces bacterial enrichment in airborne jet drops. Limnol Oceanogr 1981; 26:961 - 4; http://dx.doi.org/10.4319/lo.1981.26.5.0961
- Wendt SL, George KL, Parker BC, Gruft H, Falkinham JO 3rd. Epidemiology of infection by nontuberculous Mycobacteria. III. Isolation of potentially pathogenic mycobacteria from aerosols. Am Rev Respir Dis 1980; 122:259 - 63; PMID: 7416602
- Parker BC, Ford MA, Gruft H, Falkinham JO 3rd. Epidemiology of infection by nontuberculous mycobacteria. IV. Preferential aerosolization of Mycobacterium intracellulare from natural waters. Am Rev Respir Dis 1983; 128:652 - 6; PMID: 6354024
- Febriani Y, Levallois P, Gingras S, Gosselin P, Majowicz SE, Fleury MD. The association between farming activities, precipitation, and the risk of acute gastrointestinal illness in rural municipalities of Quebec, Canada: a cross-sectional study. BMC Public Health 2010; 10:48; http://dx.doi.org/10.1186/1471-2458-10-48; PMID: 20113516
- Wren G. Johne's in beef cattle. Bovine Veterinarian 2000; 2:4 - 7
- Williams ES, Spraker TR, Schoonveld GG. Paratuberculosis (Johne’s disease) in bighorn sheep and a Rocky Mountain goat in Colorado. J Wildl Dis 1979; 15:221 - 7; PMID: 480512
- Williams ES, Snyder SP, Martin KL. Pathology of spontaneous and experimental infection of North American wild ruminants with Mycobacterium paratuberculosis. Vet Pathol 1983; 20:274 - 90; http://dx.doi.org/10.1177/030098588302000304; PMID: 6879954
- Forde T, Kutz S, De Buck J, Warren A, Ruckstuhl K, Pybus M, et al. Occurrence, diagnosis, and strain typing of Mycobacterium avium subspecies paratuberculosis infection in Rocky Mountain bighorn sheep (Ovis canadensis canadensis) in southwestern Alberta. J Wildl Dis 2012; 48:1 - 11; PMID: 22247368
- Williams ES, DeMartini JC, Snyder SP. Lymphocyte blastogenesis, complement fixation, and fecal culture as diagnostic tests for paratuberculosis in North American wild ruminants and domestic sheep. Am J Vet Res 1985; 46:2317 - 21; PMID: 4073642
- Kaittanis C, Boukhriss H, Santra S, Naser SA, Perez JM. Rapid and sensitive detection of an intracellular pathogen in human peripheral leukocytes with hybridizing magnetic relaxation nanosensors. PLoS One 2012; 7:e35326; http://dx.doi.org/10.1371/journal.pone.0035326; PMID: 22496916
- Jeyanathan M, Boutros-Tadros O, Radhi J, Semret M, Bitton A, Behr MA. Visualization of Mycobacterium avium in Crohn’s tissue by oil-immersion microscopy. Microbes Infect 2007; 9:1567 - 73; http://dx.doi.org/10.1016/j.micinf.2007.09.001; PMID: 18062905
- Tuci A, Tonon F, Castellani L, Sartini A, Roda G, Marocchi M, et al. Fecal detection of Mycobacterium avium paratuberculosis using the IS900 DNA sequence in Crohn’s disease and ulcerative colitis patients and healthy subjects. Dig Dis Sci 2011; 56:2957 - 62; http://dx.doi.org/10.1007/s10620-011-1699-6; PMID: 21484317
- Keller J, Panter H, Layer P. Management of the short bowel syndrome after extensive small bowel resection. Best Pract Res Clin Gastroenterol 2004; 18:977 - 92; PMID: 15494290