Staphylococcus aureus infections have recently spread beyond healthcare settings into the general population. A majority of community-acquired strains are multi drug resistant (CA-MRSA) and elaborate Panton-Valentine leukocidin (PVL). Despite the potent lytic activity of PVL on immune cells, its role in pathogenesis remains inconclusive. This is likely attributed to the ability of lower, sublytic levels of PVL to activate protective, proinflammatory properties of a wide range of host cells beyond those it targets for lysis. This was highlighted in an animal pneumonia model comparing isogenic PVL+ and ∆pvl CA-MRSA strains, where animals infected with PVL-producing strains exhibited better survival. In addition to triggering cell activation for increased production of proinflammatory cytokines and antibacterial factors, there were also indications that PVL modulated the immune response by downregulating TNFα and inducing apoptosis, potentially contributing to improved outcomes of infection for the host. The continuous shift in balance between S. aureus and the host underscores the challenges in treating and predicting outcomes of infection.
Background
Staphylococcus aureus is a bacterium that causes widespread hospital- and community-acquired infections. Its success as a pathogen is attributed in part to its acquisition of resistance to optimally-effective antibiotics and to an arsenal of virulence factors designed to attack or evade every level of host immune defenses. S. aureus secretes a number of cytotoxins capable of targeted killing of select host cells. With its capacity for host cell and tissue destruction, these toxins seem to be ideal weapons for establishing and maintaining staphylococcal infections. Among the array of S. aureus toxins is a bi-component, pore-forming toxin known as the Panton-Valentine leukocidin (PVL). PVL consists of two different protein subunits, which multimerize into a β-barrel structure that inserts into target host cells, effectively creating channels in the cell membranes (see for a schematic of PVL pore formation, steps outlined in gray dashed boxes). The resulting osmotic dysregulation eventually leads to cell lysis. The cytolytic activity of PVL seems to be confined to a subset of primary human immune cells, including neutrophils, monocytes and mast cells. In ex vivo experiments using purified PVL, cytotoxic activity was demonstrated down to the nM range on target cells, suggesting that PVL is a highly potent toxin. In spite of this finding, establishing a definitive role for PVL in S. aureus pathogenesis has been anything but straightforward.
Figure 1. Schematic of PVL pore formation, and the different cellular pathways it uses to activate host cells. Steps involved in immune activation are outlined in solid boxes, while steps in pore formation are outlined in gray dashed boxes. Sublytic levels of PVL activate human neutrophils by stimulating calcium ion channels, followed by an influx of calcium into the cell. This occurs prior to PVL pore formation. PVL has also been shown to activate murine macrophages via TLR2. While sublytic PVL do not require TLR to prime human neutrophils, it has been suggested that PVL lysis of host cells releases host damage-associated molecular patterns (DAMPs) that in turn is recognized by TLR to activate human neutrophils and macrophages/monocytes.
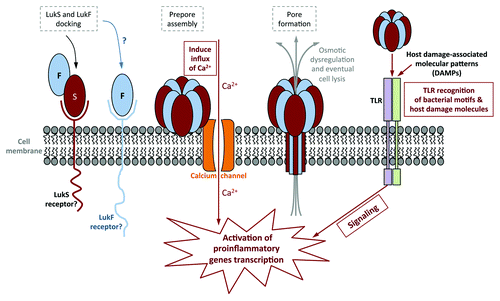
In studies where PVL was titrated below the threshold of cytotoxicity to its target cells (sublytic concentrations), not only was there no cell damage, but PVL activated those very same cells to amplify host immune defenses that could better control bacterial infection. Given that PVL elicits contradictory effects on immune cells, depending on its concentration, any potential effects it may have on staphylococcal pathogenesis could be occluded. Indeed, scientific and clinical reports on the role of PVL in infection are contradictory and highly controversial.
Role of PVL in a Model of MRSA Pneumonia
We sought to delineate the role of PVL in pneumonia by comparing isogenic wild-type (WT) and ∆pvl methicillin-resistant S. aureus (MRSA) strains in a mouse pulmonary infection model. Unexpectedly, the outcome was contrary to conventional reasoning, wherein a cytolytic toxin would be expected to enhance bacterial virulence. We found the ∆pvl strains were significantly more virulent than the corresponding PVL-producing WT parental strains, with higher mortality among the mice infected with the ∆pvl MRSA (). Consistent with those observations, increasing levels of PVL expression from the same three strains of MRSA from pvl (lukSF-PV) genes on a high copy number plasmid decreased the mortality rates and/or prolonged the time to death (). Using ELISA to measure the amounts of PVL within the lungs of infected mice, we observed a loose correlation whereby the higher the concentration of PVL detected within the lung, the lower the rate of mortality ().
Figure 2. Comparison of the virulence of PVL-producing MRSA strains with their respective isogenic ∆pvl mutants in a mouse pneumonia model. (A) Comparison of the percent mortality at 48 h in mice infected with three different MRSA strains, their ∆pvl isogenic strains and, in the case of strain MW2, the PVL-complemented strain (pvl comp). p values were determined by Chi-square analysis. Differences with strain NRS193 were not significant. (B) Survival curves comparing outcomes in mice infected with WT PVL+ MRSA or isogenic strains expressing higher levels of PVL from pOS1-pvl. p values by log-rank test. (C) Trend correlating levels of PVL production and survival in a mouse model of S. aureus pneumonia. (D) Pathology of selected murine lung sections 8 h post infection with MRSA strain MW2, and its isogenic ∆pvl mutant.
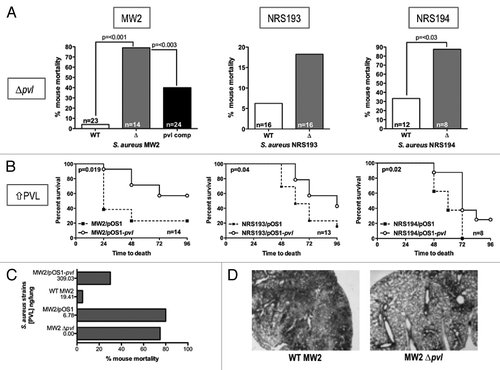
Outcomes from our pneumonia infection models indicate that PVL may have a protective role within the host, which is consistent with the ability for PVL to activate immune cells. Mouse models of infections are ideally suited to study the proinflammatory aspect of PVL, as mouse cells are relatively resistant to PVL’s lytic activity. Indeed, lung pathology from infected mice 8 h after infection showed the PVL-producing WT MW2 strain induced extensive inflammation, while inflammation was notably absent from samples infected with MW2 ∆pvl (), suggesting that PVL elicits proinflammatory reactions from mouse cells as well. The fact that WT MW2 infected mice faired considerably better than mice infected with MW2 ∆pvl would indicate that the inflammation at an early time point soon after infection was beneficial, and favored a positive outcome of infection for the host.
PVL and Immune Activation
Low, sublytic concentrations of PVL can activate primary human immune cells to secrete an array of proinflammatory cytokines, including interleukin (IL)-8 and leukotriene B4. These cytokines act as chemoattractants, further recruiting more activated neutrophils to the site of infection. Additionally, activated primary immune cells turn on an array of pathways designed to contain microbial infections, including the secretion of antimicrobial factors, and enhanced phagocytosis.
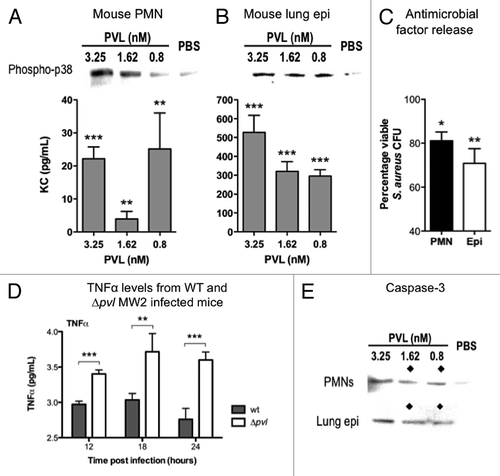
Our next aim was to dissect the basis, on a molecular level, for the differences in the survival outcomes in the pneumonia infections. To do so, we used a mouse lung epithelial cell line (MLE 12) originating from the same strain of mice used in the infection models (FVB), as well as mouse neutrophils isolated from the bone marrow of FVB mice. Cell activation was measured using three benchmarks, the secretion of the proinflammatory cytokine KC (mouse homolog of human IL-8), phosphorylation of the p38 mitogen-activated protein kinase (phospho-p38 MAPK, a signaling molecule in the phospho relay pathway leading to increased transcription of proinflammatory genes), and the release of antimicrobial factors. Both mouse neutrophils and lung epithelial cells responded by all measures to stimulation by PVL (). Despite the resistance of mouse cells to PVL cytotoxicity, these cells responded to the proinflammatory inductive activities of PVL. This is consistent with the outcome of the mouse pneumonia infections, based on the assumption that the activation of immune defenses by PVL protected mice from MRSA infections.
Figure 3. Immunomodulatory effects of PVL on mouse cells. (A and B) Detection of phospho-p38 and murine KC produced by purified PMNs from FVB mice (A), and mouse lung epithelial cells MLE12 (B) exposed to indicated concentrations of purified PVL. (C) Percentages of viable MRSA strain MW2 after addition of supernatants from the indicated cells that were first incubated with purified PVL, compared with bacterial count in cells lacking exposure to PVL. Bacterial counts from cell supernatant killing assays are averaged from a minimum of 3 independent experiments. Error bars denote SEM. Statistical analyses were performed by the t-test (***p < 0.01; **p < 0.05; *p < 0.01). (D) Detection of TNFα in murine pulmonary tissues infected with WT or Δpvl MRSA strain MW2 18 h after intranasal infection with 5 × 108 cfu/mouse. (E) Production of Caspase 3, as determined by immunoblot, from indicated cells 6 h after exposure to indicated concentration of PVL. ◆ denotes PVL concentrations that stimulate release of antibacterial factors by cells.
PVL Mediated Resolution of Inflammation
To ascertain further differences in host immune responses elicited by the WT and ∆pvl MRSA strains, pulmonary homogenates were analyzed for differential cytokine production at several time points post infection. Of the panel of cytokines tested, a meaningful difference was observed in the levels of TNFα. WT MW2 infected mice seemed to maintain a steady level of TNFα, whereas TNFα levels were significantly higher in mice infected with the ∆pvl counterpart (). It has been well documented that uncontrolled increases in TNFα can result in death arising from symptoms akin to those associated with septic shock, one of which being neutropenia, which is consistent with the absence of PMNs or inflammation in the lungs of mice infected with the ∆pvl strain. The elevated levels of TNFα could explain the increased mortality of mice infected with the ∆pvl strains. These findings would suggest that PVL could possibly be regulating the levels of TNFα at the site of infection. Consistent with that, downregulation of TNF transcripts in human PMNs exposed to sublytic concentration of PVL has been reported.
Apoptosis is a form of programmed cell death crucial to resolving inflammation. The apoptotic marker Caspase 3 was used as an indicator of cells undergoing apoptosis in response to PVL. Increased detection of Caspase 3 from mouse cells upon extended incubation (6 h) with sublytic concentrations of PVL would suggest that mouse cells became apoptotic in response to PVL (). Interestingly, we noticed an inverse correlation between the concentrations of PVL that induce apoptosis and activate cells (as measured by the release of antimicrobial factors into the supernatant). The collective data indicated that PVL can possibly both activate and downregulate inflammation, but likely only one response predominates at a time. Based on the timing of Caspase 3 induction, which occurred after detection of phospo-p38, it would appear that PVL first activated the innate immune response, followed by downregulating the inflammation in which it started.
Immunomodulatory Effects of PVL on Human Cells
Here, we confirmed prior findings that cells susceptible to PVL lysis, like human neutrophils, can nonetheless be activated by PVL, but only at sublytic levels (higher levels cause neutrophil lysis). However, PVL cytotoxicity is limited to a subset of human primary immune cells, while other cells experience little to no cell damage when exposed to PVL. Since mouse cells resistant to PVL-mediated lysis could still be activated by PVL, we wondered if human cells not normally susceptible to PVL could respond in a similar manner. To test this, a human alveolar basal epithelial cell line (A549), which is resistant to lysis by PVL, was used. The human cells did indeed respond similarly, with PVL stimulating the secretion of IL-8, phosphorylation of p38 MAPK, as well as release of antimicrobial factors (). These results suggest that host cell activation by PVL could potentially be much more far-reaching than its lytic activity, which is limited to a subset of immune cells. This may explain why MRSA infections with PVL-producing strains are often associated with better outcomes, should its beneficial proinflammatory properties outweigh its lytic effect.
Figure 4. Immunomodulatory effects of PVL on human cells. (A and B) Detection of phospho-p38 and human IL-8 produced by purified PMNs (A) and cultured A549 human alveolar basal epithelial cell line (B) exposed to indicated concentrations of purified PVL. (C) Percentages of viable MRSA strain MW2 after addition of supernatants from the indicated cells that were first incubated with purified PVL, compared with bacterial count in cells lacking exposure to PVL. Bacterial counts from cell supernatant killing assays are averaged from a minimum of 3 independent experiments. Error bars denote SEM. Statistical analyses were performed by the t-test (***p < 0.01; **p < 0.05; *p < 0.01). (D) Production of Caspase 3, as determined by immunoblot, from indicated cells 6 h after exposure to indicated concentration of PVL. ◆ denotes PVL concentrations that stimulate release of antibacterial factors by cells.
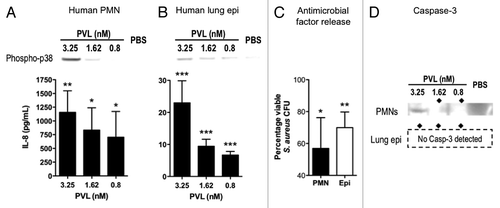
Like the mouse cells, increased levels of Caspase 3 in human neutrophils incubated with sublytic levels of PVL would suggest that these cells were undergoing apoptosis and in turn downregulating the immune response in response to PVL (). However, the human alveolar cells did not induce Caspase 3 at any amounts of PVL tested (up to 13 nM, not shown). The levels of IL-8 released by A549 cells by PVL were many orders of magnitude lower than that from human neutrophils, suggesting the possibility that low levels of proinflammatory cytokines may activate basal levels of inflammation that does not necessitate downregulating.
In the case of the murine host, whose cells are resistant to lysis by PVL, it nonetheless appeared to harness the beneficial properties of PVL during MRSA infections, as reflected by lower mortality rates of mice infected with PVL-producing strains. However, it is more challenging to envision the role PVL plays in the human host, since PVL can be detrimental or beneficial to the host, depending on the concentrations of PVL. Low, sublytic levels of PVL may serve to amplify host immunity during the initial stages of infection, where bacterial numbers and toxins elaborated by them are still low. Once an infection is established, there is conceivably a gradient of PVL, with the highest concentration near the site of infection, and a decrease in PVL as distance from the infection increases, although it is uncertain if the concentration of PVL achieved in vivo during active infection would even be sufficient to cause cell lysis ().
Implications of PVL on S. aureus Infections
Given that PVL has such potent lytic activity on primary human immune cells, which are essential in controlling infections, it is unexpected that a PVL-dependent virulence phenotype is not readily observed. The study of PVL virulence in mouse models of S. aureus infections could pose some problems because of the relative resistance of mouse cells to PVL-mediated lysis. However, mouse models aside, there is example after example in the clinical setting whereby PVL makes no contribution to pathogenesis, and even in some cases, infections with PVL+ S. aureus are associated with better infection outcomes.
The findings reported here reaffirm that PVL has strong proinflammatory capabilities that could well influence the outcome of infection in favor of the host. PVL has been shown to activate mammalian cells by at least two mechanisms: by inducing calcium influx into host cells, and via host recognition of bacterial molecular patterns by mammalian toll-like receptors (TLR) (, steps involved in immune activation are outlined with solid boxes). Both pathways trigger signal cascades that switch on transcription of proinflammatory cytokine genes and activate antibacterial mechanisms. TLRs are expressed widely on many cells, suggesting a potential means by which PVL can activate cells that are resistant to its lytic activity. Inflammatory cytokines elicited by PVL could in turn activate other cells to secrete additional cytokines, potentially augmenting inflammation exponentially. Additionally, PVL can also synergize with other S. aureus factors to amplify the host inflammatory response. Could these proinflammatory, immune-activating properties of PVL be alleviating S. aureus infection?
As is always the case, there are important caveats to the activation of inflammation. Overactive or even uncontrolled immune activation has detrimental effects on the host due to the release of toxic substances such as low pH vesicles, TNFα and reactive oxygen species. Thus, to minimize damage to the host resulting from immune activation, inflammation needs to be controlled and downregulated once the initial threat has been neutralized. Intriguingly, data garnered from our mouse infections would suggest that PVL can also downregulate inflammation, consistent with reports that human PMNs downregulated genes involved in the inflammatory response upon prolonged incubation with sublytic PVL.
In the same manner by which bacteria are constantly changing to enhance their colonization and infectivity potentials, the host may have developed the means to battle these organisms by targeting one of its most potent virulence factors. In treatment of any patient with a S. aureus infection, both the factors elaborated by the pathogen, as well as the host response to it, must be considered. Activation of innate immunity is not limited to PVL, but has also been shown to occur with α-toxin, γ-hemolysin, LukAB/GH and LukED, other toxins elaborated by S. aureus. Additionally, certain combinations of these toxins can amplify the inflammatory response even further. In light of multiple S. aureus toxins having properties potentially beneficial to the host, the use of therapeutic antibodies should be cautioned. The neutralization of S. aureus toxins, and its proinflammatory effects, could inadvertently have the opposite outcome, as evidenced previously by our group in a skin abscess infection model. Clearly, more study is needed for a better understanding of the delicate host-pathogen interaction to more effectively control the increasing cases of devastating staphylococcal infections.