Abstract
Malaria affects millions of people around the world and a small subset of those infected develop cerebral malaria. The clinical presentation of cerebral malaria differs between children and adults, and it has been suggested that age-related changes in the endothelial response may account for some of these differences. During cerebral malaria, parasites sequester within the brain microvasculature but do not penetrate into the brain parenchyma and yet, the infection causes severe neurological symptoms. Endothelial dysfunction is thought to play an important role in mediating these adverse clinical outcomes. During infection, the endothelium becomes activated and more permeable, which leads to increased inflammation, hemorrhages, and edema in the surrounding tissue. We hypothesize that post-natal developmental changes, occurring in both endothelial response and the neurovascular unit, account for the differences observed in the clinical presentations of cerebral malaria in children compared with adults.
Introduction
Malaria is a leading cause of morbidity and mortality globally with up to half of the world’s population at risk of infection. Most deaths occur in children in Africa, where malaria accounts for approximately 1 in 6 childhood deaths.Citation1 Malaria is an intravascular disease caused by Plasmodium spp and is spread by infected female Anopheles mosquitoes. The majority of malaria deaths are caused by infections with Plasmodium falciparum, but the presenting symptoms and pattern of illness vary according to transmission intensity, patient age, and host and parasite genetic determinants. The endothelium is central to malarial pathobiology as it acts as the critical barrier between host and pathogen. Endothelial activation and dysfunction in malaria is well described. In this review, we compare differences in the clinical and pathological features of cerebral malaria (CM) in children and adults, focusing on the brain endothelium and the neurovascular unit (NVU). As CM often strikes children at a critical time in brain development, maturational changes in the cerebral vasculature may account for some of the differences in disease presentation and outcome between children and adults.
Key differences between CM in children and adults include: lower mortality, more frequent seizures and more commonly reported neurocognitive sequelae, higher rates of associated anemia, and lower rates of renal dysfunction. Other relative differences include retinal vessel changes, as well as more frequent ring hemorrhages and inflammatory cell accumulation in the pediatric brain microvasculature. We hypothesize that developmental aspects of the endothelium and the NVU underlie these observations. This review therefore attempts to integrate a current understanding of the NVU and neurodevelopment with age-related differences in childhood and adult CM.
Here, we provide a narrative review of the pediatric and adult literature of CM, drawing on experimental data from animal models where it informs mechanistic understanding of disease processes. We begin with a description of the anatomy and function of the NVU, and highlight selected molecular pathways involved in endothelial activation and dysfunction. We next review the dramatic growth and changes that are occurring in the brain and brain vasculature during the period of maximum incidence of CM, in early childhood. We then integrate these findings within a framework of CM pathogenesis that includes mechanisms of parasite sequestration and dysfunction of the NVU, highlighting age-related changes in the key pathologic pathways. We review differences in clinical, ophthalmologic, and autopsy findings in children and adults with CM, drawing links between pathologic pathways of illness and age-specific disease manifestations. Finally, we propose that therapies targeting the endothelium are attractive potential adjuncts to improve outcomes in CM, and that such therapies may have to be age-appropriate in light of the differences between children and adults.
Methods
Information for this narrative overview was drawn from the following sources: MEDLINE search from 1966–May 2013 (keywords: blood–brain barrier; neurovascular unit; brain development; cerebral malaria; endothelial dysfunction; child; adult), and hand searches of references of retrieved literature. Papers were included if they provided information on brain development, the neurovascular unit, or adult–child differences in cerebral malaria, and were excluded if they did not provide insight into age-related differences in cerebral malaria.
The Neurovascular Unit
P. falciparum is an intra-erythrocytic parasite that exerts its pathogenic effect on the central nervous system (CNS) without direct contact with neurons. The interface between the vessel lumen, where the parasite resides, and the brain parenchyma, the target tissue in CM, represents a critical barrier that must be breeched in order to generate CNS injury in malaria. Thus, the endothelium and closely associated cellular components of the NVU play central roles in malaria pathogenesis.
The notion of a blood–brain barrier (BBB) originated with early experiments by Ehrlich (1885) and Goldmann (1909) that showed that the brain parenchyma, unlike other organs, was protected from dyes injected into the bloodstream.Citation2 Later investigators showed that smaller molecules, bile salts and ferrocyanide, which act as neurotoxic agents, did not penetrate the brain from the bloodstream.Citation2 These classic experiments describe a BBB that acts as a relatively impermeable blockade to macromolecule and toxin extravasation, yet the BBB is a more complex gatekeeper. Conceptually, the BBB must regulate the flow of fluid, ions, small molecules (e.g., glucose, amino acids), toxins, drugs, large macromolecules, cytokines, antibodies, and cellular components. Regulation of the bi-directional passage of these elements between the vascular and CNS interstitial compartments is governed by multiple cellular and molecular mechanisms. The current conceptual construct of the NVU is related to the BBB, but encompasses the complex interplay between numerous cellular elements, including neurons, glia, and their nutrient microvasculature, which together perform multiple functions, one of which is to regulate transport from the vascular space into the brain parenchyma.
Structure of the neurovascular unit
The NVU consists of multiple layers of cellular and extracellular components.Citation3 Proceeding radially from the blood vessel lumen, the first layer of the NVU is a glycocalyx secreted by the endothelial cells lining the vessel intima. Next, a layer of endothelial cells lie in tight apposition, sealed together by belt-like tight junctions and punctate adherens junctions. The endothelial cells are bound to their basolateral pole by the extracellular basal lamina. Pericytes are located in close proximity and communicate with adjacent endothelial cells through peg-shaped connections. The Virchow–Robin space forms a true or potential fluid zone between the endothelial basal lamina and the glia limitans, a layer of astrocytic endfeet which form a lacework of fine lamellae around the microvasculature.Citation4,Citation5 Mast cells, containing neuroactive and vasoactive substances, are located at perivascular locations on the brain side of the BBB in apposition with astrocytic and neuronal processes.Citation6
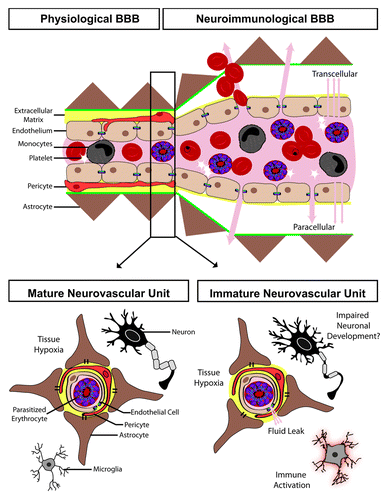
Important histological differences exist between the capillary bed and larger vessels, with functional implications for vessel barrier function. Capillaries (by definition) do not have a smooth muscle intima. Thus, the basolateral pole of the endothelium lies adjacent to pericytes embedded within the basement membrane and the astrocytic foot processes. In larger vessels (arteries and veins), the smooth muscle layer in the vessel media provides an additional barrier to molecular diffusion and cellular transit. The post-capillary venules in the CNS possess intermediate features, including mural cells, with properties of both pericytes and smooth muscle cells, as well as a perivascular space (the Virchow–Robin space). The perivascular space is patrolled by macrophages which act as phagocytes, providing an added barrier mechanism. Under inflammatory conditions, immune cells migrate first across the endothelial layer into the perivascular space, and from the perivascular space across the glia limitans into the brain parenchyma. This anatomical organization creates two functionally distinct BBBs: the physiologic BBB at the capillary level and the neuroimmunological BBB at the level of the post-capillary venules, with important implications for immunologic privilege of the CNS ().Citation7 In CM, parasitized erythrocyte (PE) sequestration occurs at the level of the post-capillary venulesCitation8 and recent experiments have shown that neurological signs in animal models of experimental CM are related to the regulated opening of fluid transport pathways at the level of the neuroimmunological BBB.Citation9
Figure 1. The effect of cerebral malaria on the blood–brain barrier (BBB) and the developing neurovascular unit. The BBB can be separated into the physiological BBB, where the endothelial cells are in close apposition to astrocyte end processes and pericytes, and the neuroimmunological BBB, which has a perivascular space separating the endothelial cells from the astrocytic foot processes. During malaria infection, the endothelium becomes activated leading to both transcellular and paracellular leak. The neurovascular unit, which makes up the BBB, undergoes marked changes during development which may impact the pathogenesis of cerebral malaria, including: synaptic pruning, myelination, immune system maturation, maturation and differentiation of glial cells, increased expression of tight junction proteins, and ultimately improved endothelial barrier function.
The anatomical basis of this barrier function lies in the tight junctions between endothelial cells and their low pinocytotic activity.Citation10 The endothelium in the brain has more complex tight junctions than in capillary beds outside the CNS, approximately 50–100 times tighter than peripheral microvessels.Citation11 The tight junctions between endothelial cells consist of several molecular components including occludin, claudins, and the zona occludens (ZO) proteins. Occludin is an integral membrane protein, found in higher concentrations within the CNS, which acts as an important determinant of BBB permeability. The distribution of occludin is continuous at tight junctions in the brain, but discontinuous at cell–cell contacts of endothelial cells outside the brain vasculature.Citation12 Occludin knockout mice display a complex phenotype, including calcifications in the brain, suggesting that occludin is essential for well-developed immunologic privilege of the brain. The claudins comprise a family of more than 20 proteins that form tight junction strands through the interaction of their extracellular loops.Citation13 Important claudins at the BBB include Claudin-5, -3, and -12.Citation14 Claudins appear to act as size-selective filters for macromolecules, as illustrated by claudin-5 knockout mice, which die as neonates due to a loosening of the BBB for molecules <800 Da.Citation14
Function of the neurovascular unit
Flux of materials across the BBB can occur via paracellular (across tight junctions) or transcellular routes. The BBB is permissive to the passage of small lipophilic molecules such as oxygen, CO2, and ethanol, which diffuse freely across the lipid membranes of the endothelium. Small polar molecules, such as glucose and amino acids, have specific transport systems. Other transporters regulate the efflux of potentially toxic metabolites out of the brain (e.g., glutamate). Exogenous compounds are actively excluded from the brain interstitium by systems such as P-glycoprotein, an energy-dependent efflux pump with broad specificity localized at the luminal endothelial membrane. Endocytosis and transcytosis occur at reduced rates in the brain endothelium relative to peripheral capillaries. Nonetheless, larger macromolecules such as peptides and lipoproteins transit to the brain via specific systems for receptor-mediated and adsorptive endocytosis. The CNS endothelium performs protective and detoxifying functions with enzyme systems such as monoamine oxidase that block circulating neuroactive agents from the central synapses. Collectively, the barrier functions of the NVU encompass a host of physiologic processes that enable the endothelium to protect and regulate the brain microenvironment.
In addition to the endothelial layer, several accessory cells contribute to the function of the NVU, including pericytes and astrocytes. Pericytes regulate endothelial transcytosis, mediate the appropriate alignment of tight junction proteins, and govern endothelial activation and quiescence by releasing angiopoietin-1 (Ang-1), a key mediator of endothelial stabilization.Citation15 Illustrating their central role in BBB function, vessel permeability is directly correlated with extent of pericyte coverage, with CNS vessels having the highest levels of coverage.Citation15 Astrocytes support the function of neuronal populations and communicate with segments of associated vasculature. Astrocyte foot processes form the glia limitans, and regulate the flux of ions and fluid across the BBB.Citation16 Both pericytes and astrocytes participate in the regulated opening of the BBB during inflammation.Citation15,Citation16
Numerous factors have been shown to affect the permeability of the BBB, including angiopoietins,Citation17 sphingosine-1-phosphate (S1P),Citation18 Slit,Citation19 nitric oxide, bradykinin, histamine, serotonin, glutamate, purine nucleotides (ATP, ADP, and AMP), adenosine, platelet-activating factor, phospholipase A2, arachidonic acid, prostaglandins, leukotrienes, interleukins (IL-1α, IL-1β, and IL-6), tumor necrosis factor (TNF), macrophage-inhibitory proteins MIP1 and MIP2, and free radicals.Citation11 Several of these mediators have been implicated in CM pathologyCitation20-Citation23 and selected pathways are discussed below and illustrated in .
Figure 2. Key molecular pathways that govern endothelial barrier function and may play a role in the endothelial dysfunction occurring during malarial infection. (1) Ang-1 mediated signaling inhibits NFκB, prevents the internalization of VE-Cadherin, promotes nitric oxide formation and promotes endothelial cell survival. Conversely, Ang-2 competes with Ang-1 for Tie2 binding after being released during Weibel–Palade (WP) body exocytosis. Ang-2 antagonizes the effects of Ang-1. (2) Nitric oxide inhibits exocytosis of WP bodies. (3) Sphingosine-1-phosphate (S1P) stabilizes proteins that make up tight junctions, adherens junctions and focal adhesions. (4) Slit-Robo4 signaling promotes VE-cadherin localization to the plasma membrane and inhibits VEGF mediated endocytosis of VE-cadherin. EC, endothelial cell; ECM, extracellular matrix.
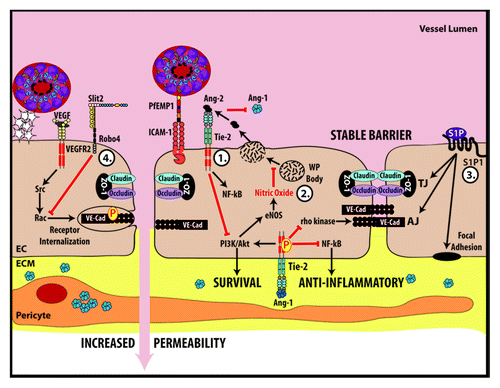
The angiopoietin–Tie2 axis represents a recently described pathway central to endothelial regulation. During systemic inflammation, Ang-1 exerts a stabilizing effect on endothelial junctions through its interaction with Tie2, halting the permeability-inducing effects of the vascular endothelial growth factor (VEGF)-mediated signaling pathway. This inhibition occurs at the level of Src, and results in decreased phosphorylation and reduced endocytosis of the adherens junction component VE-cadherin ().Citation17 Decreased levels of Ang-1, a promoter of endothelial quiescence, are associated with severe malaria and predictive of fatal outcomes.Citation24,Citation25 Low Ang-1 levels are also associated with retinopathy in severe malaria, suggesting a link to vascular injury.Citation26 Ang-2 antagonizes Ang-1 at the Tie2 receptor (). Ang-2 is released from activated endothelial cells when they exocytose Weibel–Palade bodies (WPBs), which contain a variety of molecules in addition to Ang-2, including von Willebrand Factor (vWF), P-selectin, and Endothelin-1.Citation27 Released Ang-2 sensitizes endothelial cells to TNF which results in increased expression of the intercellular adhesion molecule (ICAM)-1.Citation28 Ang-2 levels are elevated in patients with severe malaria compared with those with moderate or mild disease among adults in OceaniaCitation29 and Asia,Citation24 and in children in Africa.Citation25 Plasma levels of Ang-2 are correlated with disease severity and act as a predictor of mortality in both adults and children with malaria infection.Citation21-Citation25,Citation29-Citation31 Plasma levels of angiopoietins were not affected by age in a study of CM from India including both children and adults.Citation24 However, the angiopoietin receptor, Tie2, increases with age in young infantsCitation32 and in aging rats,Citation33 suggesting an age-dependent responsiveness of the endothelium to angiopoietin signaling.
WPB exocytosis is inhibited by nitric oxide (),Citation34 a pleiotropic signaling molecule that has been shown to have decreased bioavailability in severe malaria.Citation35-Citation37 Reactive hyperemia-peripheral arterial tonometry (RH PAT) index, a physiologic marker and surrogate for measuring nitric oxide bioactivity, is negatively correlated with Ang-2.Citation29 Nitric oxide also decreases endothelial expression of key adhesion molecules including ICAM-1.Citation38 The importance of nitric oxide during severe malaria was further supported by the use of inhaled nitric oxide as an adjunctive therapy for the treatment of experimental CM.Citation20 It was shown that inhaled nitric oxide resulted in decreased expression of endothelial activation markers, decreased inflammation, decreased endothelial permeability and increased survival in mice with experimental CM.Citation20 Thus endogenous nitric oxide signaling appears to be an important regulatory molecule in endothelial activation. Age-dependent changes in nitric oxide synthesis have been described,Citation39 suggesting that further studies into this pathway may shed light into differences in pediatric and adult CM.
Sphingosine-1-phosphate (S1P) is a signaling lipid molecule involved in numerous cellular processes including inflammation and endothelial homeostasis through its interaction with G-protein-coupled receptors. Downstream signaling results in stabilization of the tight junction protein ZO-1 ().Citation40 S1P has been proposed to play a protective role in human and experimental CM.Citation18 Agonists at the S1P receptor stabilize the BBB and improve outcomes in experimental CM.Citation9,Citation18 S1P also plays an important role in neurogenesis, endothelial proliferation and morphogenesis, angiogenesis, and promotes CNS myelination through interactions with oligodendrocytes.Citation41 Given these important functions in the development of the NVU and in brain myelination, S1P may be an interesting target to dissect age-related differences in CM.
Roundabout (Robo) receptors and their Slit protein ligands were originally discovered in the context of brain development as mediators of repulsive axon guidance signaling.Citation42 In addition to neural development, a number of other functions have been described, and an endothelial-specific Robo family member, Robo4, mediates endothelial stability and prevents excessive destabilization in the presence of circulating cytokines. The mechanism of action involves Slit-Robo4 mediated inhibition of (cytokine-induced) phosphorylation of VE-cadherin at a key tyrosine residue, resulting in stabilization of its interaction with p120-catenin, and localization to the plasma membrane ().Citation19 Robo4-dependent Slit signaling improves survival in experimental models of sepsis and influenza infection,Citation19 and may play a role in other infectious diseases characterized by a cytokine storm, such as CM. Given roles in CNS and vascular development, the Slit–Robo signaling pathway may operate differentially in pediatric and adult CM, and might explain some of the phenotypic differences in disease manifestations.
In summary, the endothelium plays a critical barrier function which is regulated through several pathways which include the Ang–Tie2 axis, nitric oxide, S1P signaling, and the Slit–Robo pathway. In reviewing these molecular mechanisms (illustrated in ), we note a paucity of data comparing children and adults, and/or juvenile vs. mature rodents in model systems, and we propose that these cellular programs represent fruitful avenues of investigation to dissect age-dependent differences in CM.
Development of the Central Nervous System and the Neurovascular Unit
CM in hyper- and holo-endemic areas affects primarily children under five years of age,Citation1 coinciding with a period of rapid brain growth and physiologic changes in the BBB. Here, we briefly review the development of the CNS and the NVU and postulate how this might alter disease manifestations in patients of different ages.
Development of the CNS proceeds along a complex and ordered timeline that begins with the formation of the neural tube around the third week of gestation, and continues throughout childhood and adolescence.Citation43 During the embryonic and fetal stages in utero, brain structures emerge, governed by the proliferation, migration, and regression of neurons, glia, and vessels. Induction of the BBB occurs during embryogenesis as endothelial cells invade the CNS, and pericytes are recruited to the nascent vessels. Astrocyte–endothelial interactions also promote endothelial barrier function in vitro,Citation11 yet astrocytes do not appear until 1 week after the formation of the BBB in mice.Citation15 By birth, neuronal proliferation and migration is largely complete, brain structure and major pathways are established, and the BBB is functional to exclude macromolecules.Citation44 Brain development continues for an extended period postnatally. In the first 5 y of life, the brain undergoes a rapid increase in size, quadrupling in volume to reach approximately 90% of its final adult size.Citation45 Important postnatal changes include glial cell development and differentiation, progressive myelination of white-matter, and synaptic pruning.
Post-natal glial cell differentiation has direct implications for the NVU. The main period of differentiation of astrocytes and the encirclement of capillaries occur in rodents in the first 3 weeks of postnatal life, equivalent in terms of brain maturity to the period of early childhood in humans. Expression of astrocyte markers, glial fibrillary acidic protein (GFAP), Aquaporin-4, and S-100β, increases in the post-natal period, illustrating functional maturation during a critical window of vulnerability for inflammatory injury.Citation46 Aquaporin 4 has been shown to be upregulated with age in both the cerebral and cerebellar cortices in mice.Citation47 Therefore, reduced levels of the astrocyte water transporter aquaporin-4 may have important functional implications for BBB function in young children. Reactive astrocytes restrict inflammation, limit tissue degeneration, and preserve function after CNS injury.Citation48 The significant neurocognitive sequelae described in CM survivorsCitation49,Citation50 may be partly explained by astrocyte immaturity at the time of childhood CM.
Early in pre-natal neuronal development, exuberant growth and migration of neuronal precursors results in numerous dendritic ramifications with large numbers of synaptic connections in an extensive topographic distribution.Citation51 From birth through childhood and into early adolescence, this extensive but diffuse neuronal connectivity is pruned back, in favor of more precise contacts between neurons and their targets. Thus, the number of synapses reaches a peak of nearly twice adult levels during the pre-school years,Citation52 and then gradually declines to adult levels throughout later childhood and adolescence.Citation53 During childhood, rapid myelination and growth of the CNS white matter occurs.Citation43 It is conceivable that during this time of delicate fine-tuning of the neuronal circuitry and rapid myelination, a major inflammatory insult such as CM could have distinct repercussions for neuro-psychiatric function. It has been suggested that dysregulation of synaptic pruning, due to elevated levels of complement activation during malaria infection, may result in neurocognitive impairment in exposed children.Citation54
Development of brain endothelial tight junctions continues beyond birth in rodents. Occludin expression changes during mammalian development, increasing from low levels in rat brain endothelial cells at postnatal day 8 to higher levels at post-natal day 70.Citation12 We speculate that age-related differences in occludin expression at brain tight junctions may also account for some of the observed permeability of the pediatric neurovasculature, although this has not yet been demonstrated in humans.
Age-dependent susceptibility to brain injury
Maturation of brain elements during the pre-school years may have important implications for CNS injury in childhood. The developing brain, in contrast to the adult brain, appears to be more susceptible to a variety of traumatic, ischemic, toxic, and inflammatory insults. Ischemic and traumatic brain injury produce more profound sequelae in young victims,Citation55,Citation56 with animal models demonstrating age-related differences in BBB regulation and inflammatory infiltrates.Citation57,Citation58 The developing CNS is also exquisitely sensitive to toxin-mediated injury (e.g., methylmercury, lead, anticonvulsants, and ethanol), with vessel barrier function, astrocytes, and oligodendrocytes playing central roles in the pathogenesis.Citation59
With potentially significant implications for pediatric CM, the cerebral vasculature of juvenile vs. mature mammals appears to differ in its response to inflammatory challenge. The concept of an age-specific window of susceptibility to neuro-inflammatory injury has evolved based on a series of experiments involving an inflammatory challenge administered to animals at various ages. Juvenile, but not neonatal, mice exhibited prolific neutrophil and mononuclear recruitment whereas adult mice showed a restricted phagocyte response in response to lipopolysaccharide (LPS) injection into the brain.Citation60 Likewise, intraparenchymal injection of pro-inflammatory IL-1β in juvenile rats gave rise to a large increase in BBB permeability and recruitment of neutrophils around the injection site, whereas no increase in vascular permeability or leukocyte recruitment was observed in adult rats.Citation61 This age-dependent inflammatory infiltrate was related to increased sensitivity to the chemotactic effects of CXC chemokines in the juvenile brain.Citation62 In further experiments, young rats (<20 d old) and opossums between post-natal age 35–60 d exhibited transient permeability of the BBB to plasma proteins following systemic administration of LPS. In contrast, the BBB of older rats and opossums was refractory to the inflammatory insult.Citation63 These experiments generate intriguing hypotheses that warrant testing in experimental models of CM: the immature rodent neurovasculature may also be exquisitely sensitive to parasite-induced inflammation, resulting in exaggerated BBB dysfunction in younger animals. If supported by evidence in a rodent model, this finding might explain differences in BBB permeability, inflammatory infiltrates, and neurocognitive sequelae observed in children with CM relative to adults.
Cerebral Malaria and the Neurovascular Unit
The pathologic processes leading to convulsions, coma, and death in CM are complex and remain to be fully elucidated. Nonetheless, a unified framework that summarizes many of the diverse findings from human and animal studies views the genesis of CM as an interplay between mechanisms of sequestration, inflammation, and hemostasis leading to dysfunction of the NVU.Citation64 The endothelium plays a central role in these processes as the site of PE sequestration as well as the mediator of fluid extravasation, which may play a role in CM pathogenesis. We next focus on selected aspects of endothelial dysfunction in CM, highlighting age-dependent changes in these critical pathways.
Cytoadherence of parasitized erythrocytes and inflammatory cells to the endothelium
During infection with Plasmodium sp. the endothelium undergoes functional changes, and becomes “activated”. In the activated state, endothelial cells express a variety of cellular adhesion molecules, including: ICAM-1,Citation29,Citation65-Citation67 vascular cell adhesion molecule-1 (VCAM-1),Citation65,Citation67 and E-selectin.Citation65 The expression of these adhesion molecules by endothelial cells promotes the binding of inflammatory cells,Citation28 such as neutrophils and monocytes/macrophages, and the cytoadherence of PEs.Citation68-Citation70
Sequestration of PEs at the endothelial barrier in the brain is a central pathologic feature in CM in patients of all ages. Autopsy studies performed over the past century have established this classical mechanism of CM pathogenesis in fatal cases.Citation71,Citation72 Ultrastructural studies of fatal CM provide evidence in vivo of tightly packed capillaries and venules, with close apposition of the red blood cell and endothelial membranes.Citation8 Electron-dense surface knobs on the PE surface appear to mediate binding to the endothelium.Citation8 The mechanism of cytoadherence of P. falciparum infected erythrocytes to the endothelium involves interactions between surface molecules expressed by the parasite and exported to the RBC membrane, such as P. falciparum erythrocyte membrane proteins (PfEMP1), and endothelial cell receptors such as ICAM-1. Interestingly, it has recently been suggested that the increased effectiveness of artesunate compared with other anti-malarials may be due in part to its effects on the endothelium.Citation69 Experiments in vitro demonstrated that treatment with artesunate is associated with decreased expression of ICAM-1 and therefore leads to decreased adhesion of PEs.Citation69
Changes in the ability to increase expression of adhesion molecules after an inflammatory insult may be altered with age. In response to radiation injuryCitation73 or bacterial infection,Citation74 rodents of increasing age had attenuated increases in ICAM-1 expression compared with their younger counterparts. In another study, mice primed with TNF, injected into the ventricle, had increasing ICAM-1 induction with increasing age.Citation75 However, directly injecting TNF may create an artificial upregulation of ICAM-1 expression with age. Following an inflammatory insult, older mice have been shown to have decreased levels of TNF mRNA expression compared with younger mice.Citation73 These findings suggest that children may have a greater ability than adults to upregulate expression of ICAM-1 in response to malaria infection, and, therefore, have increased PE sequestration within the cerebral vasculature and increased neurological injury. However, further studies are required in order to elucidate age-related changes in the complex inflammatory response that occurs in the context of CM.
In rodent models of neuro-inflammation, reduced leukocyte accumulation is seen in response to cytokine and chemokine challenge in adult compared with juvenile animals. In these studies, exaggerated neutrophil accumulation in young rodents after administration of IL-1β and CXC chemokines was associated with increased leak across the BBB.Citation61,Citation62 This is consistent with findings from autopsy studies of fatal malaria, in which pediatric series report leukocyte accumulation in the microvasculature more than adult series.Citation72 Such observations may explain in part the relative sensitivity of the pediatric CNS to CM.
Dysfunction of the neurovascular unit
A decrease in endothelial integrity has been well documented in murine models of malaria by the measurement of Evan blue leak into various organs including the brain, lungs and kidneys.Citation20,Citation67,Citation76-Citation80 There are three main mechanisms that increase the permeability of the endothelial layer: paracellular leak, transcellular leak, and leak due to endothelial cell death. The majority of the current literature investigating endothelial dysfunction in experimental malaria focuses on both paracellular leakage and increased permeability due to cell death. These changes in endothelial permeability are caused by a variety of factors, both host- and parasite-derived. Below we discuss a select number of molecules involved in the regulation of endothelial permeability during malaria infection and age-related changes in the endothelial response.
Paracellular leak
Tight junctions between brain endothelial cells play a key role in maintaining an effective barrier to paracellular fluid flux. PEs have been associated with changes in the expression levels of proteins that make up these tight junctions. Experiments using endothelial monolayers co-cultured with PEs from CM patients have shown decreased mRNA transcripts for tight junction proteins.Citation81 Others have demonstrated a decrease in trans-endothelial electrical resistance of human brain endothelial cell monolayers on direct contact with PEs or P. falciparum culture supernatant.Citation82 Metabolic processes of PEs are able, in a dose dependent manner, to decrease the pH of culture media to an extent that results in increased endothelial permeability.Citation68 This increased permeability is associated with a rearrangement of ZO-1, including re-localization away from the plasma membrane, resulting in gap formation. ZO-1 rearrangement was also observed in correlation with increased permeability during the incubation of parasitic histones with endothelial cells.Citation66 Parasitic histones are released from parasites undergoing schizogony and it has been shown that patients admitted to hospital with severe malaria have higher levels of circulating nucleosomes, of either host or parasite origin, than those admitted with uncomplicated malaria or severe sepsis.Citation66 In children with CM, focal loss of immunostaining has been observed for the endothelial tight junction proteins ZO-1, occludin, and vinculin when spatially associated with PEs, but without evidence of fibrinogen extravasation.Citation83 Similarly, in Vietnamese adults with fatal CM, loss of tight junction proteins was observed, with associated leakage of plasma proteins across the microvasculature.Citation84 Decreased cerebral protein expression of occludin, but not ZO-1,Citation85 and increased endothelial expression of occludinCitation12 in rats with aging suggests that there are changes in the composition of tight junction proteins that occur with the aging process, and further research is required to elucidate the impact of this on CM pathogenesis.
Tight junction breakdown, leading to increased paracellular leak, can be initiated by the activity of matrix metalloproteinases (MMPs), which act to cleave tight junction proteins. MMPs target and degrade structural proteins of the basal lamina such as fibronectin, laminin, and heparan sulfate, and tight junction proteins such as occludin, ZO-1, ZO-2, and claudin-5.Citation2 The loss of tight junctions can contribute to the opening of the BBB during ischemic and inflammatory insults. Involvement of MMPs has been specifically implicated in malaria. When PEs are co-cultured with endothelial cells in vitro, the endothelial cells release MMP-9Citation86 and this is associated with increased endothelial permeability to fluorescent dextran.Citation87 In contrast to what is expected, both the mRNA and activity of MMP-9 were shown to be increased with age in primary rat tenocytes and this increase was associated with decreased levels of the MMP inhibitiors, tissue inhibitor of metalloproteinase (TIMP)-1 and TIMP-2.Citation88 However, those changes occurred in tenocytes and this may not be reflective of what is occurring at the BBB in response to an inflammatory stimulus.
Endothelial cell death
In addition to increased paracellular leak, endothelial cell death also plays a role in the increased endothelial permeability observed during experimental malaria models. During infection, red blood cells undergo hemolysis which results in the release of hemoglobin and eventually cytotoxic free heme.Citation89 Free heme is cytotoxic when it interacts synergistically with a variety of compounds, including TNF,Citation90 LPS,Citation91 reactive oxygen species,Citation92 and low density lipoprotein (LDL).Citation93 Free heme is degraded by heme oxygenase-1 (HO-1) into carbon monoxide, biliverdin and iron. During severe malaria infection, mice deficient in HO-1 had increased mortality compared with wild-type mice.Citation90 This was further confirmed by demonstrating that the induction of HO-1 via treatment with either nitric oxideCitation20,Citation94 or lovastatin,Citation76 or treatment with heme degradation products such as carbon monoxideCitation89 was protective in murine severe malaria. Evidence for age related changes in HO-1 have also been shown. In the rat carotid body HO-1 expression was decreased with increasing age during normoxia, and the ability to induce expression of HO-1 in response to hypoxia was attenuated with age.Citation95 On the other hand, increasing levels of HO-1 was observed with age in rat livers.Citation96 Therefore changes in HO-1 might be organ dependent and further investigation is warranted.
The adhesion of PEs to endothelial cells is able to induce apoptosis in endothelial cells in vitroCitation97,Citation98 and increased caspase-dependent apoptosis is seen in murine CM.Citation67 Treatment with atorvastatin is able to decrease the amount of cell death occurring when endothelial cells are co-incubated with PEs,Citation99 and is able to prolong survival in experimental CM.Citation100 Mice deficient in the platelet activating factor receptor (PAFR) have improved survival from experimental CM and have marked decreases in caspase activation.Citation67 Increased apoptosis in renal cells during a murine model of malaria induced acute kidney injury was associated with increased endothelial permeability.Citation77 Recently it has been demonstrated that non-adhesion parasite factors can also stimulate cell death. Histones are able to induce caspase-independent cell death when incubated with endothelial cells,Citation66 and patient sera are also able to induce endothelial cascade-dependent apoptosis.Citation101
Endothelial dysfunction, in terms of both cytoadherence and increased permeability, play key roles in the molecular pathology of CM. Due to limited experimental data, it is important to explore age-related changes in the endothelium that occur during CM and how this contributes to disease pathogenesis. Further understanding of the dysfunction at this level may inform our understanding of the clinical presentation of CM and why it differs between children and adults. This knowledge may lead to the development of novel adjunctive therapies that are used for age-specific treatment of CM.
Cerebral Malaria: Age-Related Differences
Having reviewed the NVU, developmental aspects of its structure and function, age-related susceptibility of the pediatric CNS to a variety of neurological insults, and the pathological implications of endothelial dysfunction, we next turn to the bigger picture and describe age-related differences in the clinical manifestations of CM.
Clinical features
The clinical picture of severe malaria differs in children and adults, and we will focus primarily on the differences occurring during CM (). An illustration of these differences comes from two large, multi-center randomized controlled trials, one involving primary adults in Asia,Citation102 the other focusing on African children.Citation103 Together, these two studies comprise nearly 80% of all patients ever enrolled in randomized controlled trials of severe malaria.Citation103 A limitation of this comparison is that there may be important environmental, immunologic and genetic confounders between the studies.
Table 1. Contrasting pediatric and adult cerebral malaria
Despite optimal treatment with intravenous artesunate, mortality is higher in adults compared with children for severe malaria (15%Citation102 vs. 8.5%Citation103) and CM (30%Citation102 vs. 18%Citation103), in adults and children, respectively. Mortality in severe malaria increased in a step-wise manner from 6.1% in children (age < 10 y) to 36.5% in older adults (age > 50), and age was an independent risk factor for mortality.Citation104 Although the mortality rate is higher in adults with severe malaria, children under the age of 5 contribute to the highest number of deaths due to malaria globally.Citation105
CM is defined as unrousable coma without alternative explanation in the presence of peripheral parasitemia, and may occur in both children and adults. CM in adults generally occurs in the context of multi-system disease with a higher proportion of patients displaying jaundice and renal failure, whereas children may present with a more isolated CNS phenotype. Analysis of a large study involving children and adults with severe malaria using uniform inclusion criteria documented a decrease in the incidence of convulsions with increasing age.Citation104 Seizures in CM are associated with long-term structural brain abnormalities including brain atrophy.Citation106 Neurologic sequelae are reported more frequently at the time of hospital discharge in children (3.5%)Citation103 vs. adults (1%),Citation102 but often resolve over time. However, persistent cognitive deficits may develop following recovery from CM in children.Citation107-Citation110 In prospective pediatric studies from Uganda, approximately 1 in 4 children with CM had cognitive deficits 6 mo and 2 y after discharge.Citation111 Deficits were primarily in the domains of attention and working memory. Similarly, one study from Malawi showed that approximately one-third of survivors of childhood CM had neurologic abnormalities, including epilepsy, gross motor, sensory, or language deficits.Citation49
Paradoxically, pediatric patients with CM have a lower mortality rate, yet neurocognitive sequelae are more commonly reported in children. It should be noted that there is a paucity of studies on cognitive outcomes in adult survivors, such that a direct comparison of CNS sequelae between adults and children is limited by the data currently available. However, if neurocognitive sequelae are more common in children, this may suggest a heightened susceptibility to neurologic injury in younger patients which may be due to the developmental state of the brain and NVU, as described above. Alternatively, increased survival in the setting of severe systemic disease may lead to a higher proportion of brain-injured survivors in pediatric compared with adult cohorts.
Retinopathy
The pathologic hallmark of CM, sequestration of PEs in the microcirculation of the brain, is difficult to assess in a living patient. However, the retinal vasculature is structurally and functionally similar and contiguous to that of the CNS and provides an accessible window to vessels that mirror those of the brain. The spectrum of retinal signs in CM consists of four components: retinal hemorrhages, papilledema, retinal whitening, and vessel color changes.Citation112
Several studies have documented the retinal findings in African childrenCitation113-Citation117 and Asian adultsCitation118-Citation120 with CM. The prevalence of these findings is summarized in , based on three studies involving adult patients with CM from India and Bangladesh (n = 214,Citation121 n = 75,Citation122 n = 20Citation123) and two of the largest studies in children from Malawi (n = 278,Citation124 n > 1000Citation125). Commonalities between pediatric and adult patients include retinal hemorrhages, the most easily visible manifestation of malaria in the retina, present in similar proportions of children and adults. Retinal hemorrhages correlate with disease severity and with cerebral hemorrhages at autopsy in the cerebral microvasculature,Citation126 and indicate frank breech of the BBB resulting in erythrocyte extravasation. Papilledema is observed less commonly in both children and adults, and is not specific to malaria, but reflects increased intracranial pressure and portends a poor prognosis in children.Citation124,Citation127 Retinal whitening is due to reversible cytotoxic edema in response to tissue hypoxia, caused by sequestered PEs.Citation112 Fluorescein angiography studies of the retina in children with CM demonstrated areas of impaired perfusion in 82% of cases, suggesting that hypoxia and ischemia are important components in the pathogenesis of CM.Citation128 Papilledema and retinal whitening, like retinal hemorrhages, occur at similar frequencies in pediatric and adult CM patients.
A major difference between children and adults is the presence of vessel discoloration in children (20–32% of patientsCitation124,Citation125), which is not seen in adults.Citation122,Citation123 Orange or white discoloration of the retinal vessels has been attributed to dehemoglobinization of stationary erythrocytes infected with mature parasites, and is a unique feature that is highly specific for encephalopathy of malarial etiology in the pediatric population.Citation129 Depletion of erythrocyte hemoglobin may be related to local obstruction to blood flow and pronounced microvascular obstruction, and may indicate that cytoadherence of PEs, leukocytes, platelets and fibrin strands may have heightened significance in the pathogenesis of CM in children relative to adults. Alternatively, the increased incidence of systemic anemia in pediatric severe malaria relative to adults may account in part for this observation.
Autopsy findings
Autopsy studies of children who die of clinically-defined CM reveal three basic patterns of pathology: (1) sequestration of PEs, parenchymal ring hemorrhages (RHs), and intravascular accumulation of pigmented white cells and thrombi; (2) sequestration of PEs without evidence of intravascular or peri-vascular pathology; and (3) no sequestration.Citation71 With respect to the latter group of children without sequestered PEs in the brain, it should be noted that the clinical definition of CM is non-specific and as many as 23% of the children with this pre-mortem diagnosis actually have an alternative explanation for their death.Citation71
Other findings in pediatric autopsy series include axonal and myelin damage, fibrinogen extravasation, intra-parenchymal RHs and vascular thrombosis in the cerebral and cerebellar white matter and brainstem.Citation72 These findings were more pronounced in areas with prominent PE sequestration.Citation72 Monocytes with intracellular hemozoin accumulated within microvessels but were not present in the adjacent brain parenchyma.Citation72 Together, these findings point to linked pathologic processes of sequestration of PEs, intravascular accumulation of inflammatory cells, myelin damage, axonal injury, and BBB dysfunction as key pathologic processes in pediatric CM.
Adult autopsy series document noteworthy similarities and differences in neuropathology compared with younger patients. As in children, post-mortem findings in adults include: tight packing of capillaries and venules with PEs, adherence to the endothelium through knob-like projections on the red blood cell surface,Citation8 axonal injury,Citation130 and BBB dysfunction.Citation131 A detailed ultrastructural study of post-mortem specimens obtained from 7 adults with fatal CM in Thailand demonstrated an absence of inflammatory cells and platelets,Citation8 in contrast to findings in pediatric series. However, in another autopsy studies involving 23 clinically-defined CM fatalities in India, there was documented margination of mononuclear cells in a significant proportion of patients.Citation132 In this study, marginated mononuclear cells were seen in association with sequestered erythrocytes.Citation132 These tended to have a peripheral distribution within the vessel, as though adherent to the endothelial lining.Citation132
Cerebral ring hemorrhages
Overt vessel wall disruption, capillary necrosis, associated with intraluminal thrombosis and/or RHs occurs focally in fatal CM in both childrenCitation72 and adults.Citation133 RHs appear to be common in pediatric series from Malawi (30/37 patientsCitation72 and 18/24 patientsCitation71). In contrast, RHs were not observed in 7 adult patients from Thailand,Citation8 in only 3/23 adults from India,Citation132 and in 9/19 from Burma.Citation133 RHs may therefore be more common in children than adults, although methodological differences between studies (patient selection and tissue sampling method) might also account for some of these differences.Citation8 Increased vessel fragility is a recognized characteristic of the immature brain. Frank breech of the vessel wall and hemorrhage into the parenchyma is relatively common in the premature neonate, occurring at a rate of 1.45% of live births.Citation134 Germinal matrix hemorrhage may be related in part to intrinsic fragility of the immature vessel wall, coupled with fluctuations in intravascular hydrostatic pressure.Citation135 Luminal factors (e.g., tight packing of cytoadherent cellular elementsCitation8) or factors intrinsic to the vessel wall may also operate in pediatric CM to produce RHs more commonly than in adults.
Cerebral edema
Clinical, imaging, and pathology studies provide a body of evidence implicating cerebral edema as a pathologic feature of CM. However, data from adult and pediatric studies are variable, and attempts to treat cerebral edema by administration of mannitolCitation136 and dexamethasoneCitation137,Citation138 have not improved outcomes in trials to date. Thus, the significance of cerebral edema as a cause of coma and mortality remains unclear.
Increased intracranial pressures, measured by manometry of the cerebral spinal fluid (CSF), have been documented in both adultsCitation139 and childrenCitation140-Citation142 with CM. Clinical features of brainstem, uncal, and/or cerebellar herniation, as a complication of extreme intracranial hypertension, have been described in children.Citation140,Citation143 Imaging studies have also shown brain swelling. In one prospective series of 120 children, approximately half of the children had evidence of moderate-to-severe increased cerebral volume.Citation143 Similarly, brain swelling on CT scan was observed in 63% of adults in a large series of 120 patients in India.Citation139 In contrast, MRI showed no findings of cerebral edema in the majority (22/24) of adult patients in an Asian study.Citation144 Consistent with the above, brain weight is increased in fatal CM in children. Brain weight was increased in 86% of children with severe pathology (PE sequestration, intravascular and extravascular pathology), 70% of patients with sequestration alone, and 62% of patients with no sequestration.Citation72 In children, increasingly severe pathologic features on autopsy (PE sequestration, intravascular and extravascular pathology) are associated with progressive increase in brain edema. On the other hand, in the prospective study of Asian adults with CM, 2/24 patients had brain swelling or herniation associated with respiratory failure and brain death.Citation144 Increased brain swelling in adults seen on imaging did not correlate with the depth of coma or mortality.Citation139 A study of fatal CM in 20 adult Vietnamese patients documented variable occurrence of cerebral edema and plasma protein leakage. These findings did not correlate with pre-mortem coma, suggesting that cerebral edema is neither sufficient nor necessary to produce altered consciousness in adult malaria.Citation145 In summary, cerebral edema is reported in a variable proportion of pediatric and adult patients with CM, although its significance as a pathogenic mechanism remains unclear.
The cerebral edema occurring during CM may be due to cytotoxic or vasogenic causes. Dysfunction of the sodium-potassium ATPase ion pump due to hypoxia or nutrient deprivation may lead to osmotic fluid redistibution across the cell membrane and accumulation of intracellular water in neurons or glial cells. Ischemic or hypoxic insults due to mechanical effects of microvascular obstruction, as well as nutrient “steal” by local metabolically active parasites could account for cytotoxic edema in CM. Alternatively, irreversible destruction of the endothelial lining is likely one mechanism leading to vasogenic cerebral edema.Citation72 Movement of fluid from the intravascular space into the brain interstitium due to BBB dysfunction could lead to intercellular (vasogenic) edema. Finally, an increase in intravascular fluid volume within the brain due to sludging of blood flow with sequestration of P. falciparum infected erythrocytes could cause brain swelling.
Adjunctive therapy directed at reducing cerebral edema as a putative pathogenic mechanism in CM has not demonstrated benefit in clinical trials. Mannitol, an osmotic diuretic agent to reduce brain swelling, did not improve outcomes in African children with CM,Citation136 although this small trial may have been under-powered to detect a true effect.Citation146 Likewise, mortality was not reduced and duration of coma was in fact prolonged in adult patients in India who received mannitol as adjunctive therapy.Citation139 Similarly, older studies investigating the use of dexamethasone to reduce cerebral edema did not demonstrate clinical efficacy.Citation137,Citation138
Conclusions: Endothelium-Targeting Therapies Tailored to Pediatric Populations
Given the central role of the endothelium in CM pathogenesis, insights into specific aspects of pediatric disease may lead to the development of new endothelium-targeting adjunctive treatments tailored to the specific needs of children. If indeed pediatric CM follows distinct pathologic pathways, and sick children are not just “little adults”, drug development efforts could focus on this unique patient population, rather than simply translating findings from trials first performed in adults. Children appear to be particularly susceptible to damage at the BBB and stand to benefit from adjuvant therapies that stabilize the endothelium during CM.
Several therapeutics that have been successful in mouse models of severe malaria have been shown to target endothelial dysfunction, including (many of which have been mentioned above): a PAFR antagonist (UK-74,505),Citation67 various statins including atorvastatinCitation100 and lovastatin,Citation76 activated protein C,Citation101 carbon monoxide,Citation89 and nitric oxide.Citation20 Based on the experimental benefits of nitric oxide on the endothelium described above, our group is currently conducting a clinical trial in Uganda using inhaled nitric oxide as an adjunctive therapy to treat children with CM.Citation147,Citation148 Not only are adjunctive therapies that target the endothelium likely to improve the acute outcomes of CM infection but they also have the potential to improve the long-term neurological consequences of CM.Citation149 Given the enormous burden of pediatric malaria, continued efforts to explore new potential therapeutics that target the childhood response to severe malaria, particularly endothelial dysfunction, offer the promise of reducing child mortality and neurocognitive morbidity on a global scale.
| Abbreviations: | ||
| CM | = | cerebral malaria |
| NVU | = | neurovascular unit |
| CNS | = | central nervous system |
| BBB | = | blood–brain barrier |
| ZO | = | zona occludens |
| PES | = | parasitized erythrocytes |
| ICAM-1 | = | intercellular adhesion molecule-1 |
| Ang | = | angiopoietin |
| S1P | = | sphingosine-1-phosphate |
| VEGF | = | vascular endothelial growth factor |
| WPBs | = | Weibel–Palade bodies |
| vWF | = | von Willebrand factor |
| Robo | = | roundabout |
| VCAM-1 | = | vascular cell adhesion molecule-1 |
| TNF | = | tumor necrosis factor |
| GFAP | = | glial fibrillary acidic protein |
| MMPs | = | matrix metalloproteinases |
| RH PAT | = | reactive hyperemia-peripheral arterial tonometry |
| TIMP | = | tissue inhibitor of metalloproteinase |
| LPS | = | lipopolysaccharide |
| LDL | = | low density lipoprotein |
| HO-1 | = | heme oxygenase-1 |
| PAFR | = | platelet activating factor receptor |
| RHs | = | ring hemorrhages |
| CSF | = | cerebral spinal fluid |
Acknowledgments
This work was supported by Global Alliance to Prevent Preterm Birth and Still birth and Grand Challenges in Global Health: Preventing Preterm Birth Initiative Grant No. 12003 (to KCK); the Canadian Institutes of Health Research (CIHR) MOP-115160 and 13721 (to KCK), CIHR Post-PhD Fellowship (to ALC), CIHR MD/PhD Studentship Award (to REE), CIHR Clinician Scientist Phase 1 Award (to MH), and Canada Research Chair in Molecular Parasitology (to KCK).
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- World Malaria Report WHO.. . 2012.
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron 2008; 57:178 - 201; http://dx.doi.org/10.1016/j.neuron.2008.01.003; PMID: 18215617
- McAdams RM, Juul SE. The role of cytokines and inflammatory cells in perinatal brain injury. Neurol Res Int 2012; 2012:561494; http://dx.doi.org/10.1155/2012/561494; PMID: 22530124
- Kacem K, Lacombe P, Seylaz J, Bonvento G. Structural organization of the perivascular astrocyte endfeet and their relationship with the endothelial glucose transporter: a confocal microscopy study. Glia 1998; 23:1 - 10; http://dx.doi.org/10.1002/(SICI)1098-1136(199805)23:1<1::AID-GLIA1>3.0.CO;2-B; PMID: 9562180
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 2009; 31:497 - 511; http://dx.doi.org/10.1007/s00281-009-0177-0; PMID: 19779720
- Khalil M, Ronda J, Weintraub M, Jain K, Silver R, Silverman AJ. Brain mast cell relationship to neurovasculature during development. Brain Res 2007; 1171:18 - 29; http://dx.doi.org/10.1016/j.brainres.2007.07.034; PMID: 17764664
- Owens T, Bechmann I, Engelhardt B. Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 2008; 67:1113 - 21; http://dx.doi.org/10.1097/NEN.0b013e31818f9ca8; PMID: 19018243
- MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 1985; 119:385 - 401; PMID: 3893148
- Nacer A, Movila A, Baer K, Mikolajczak SA, Kappe SH, Frevert U. Neuroimmunological blood brain barrier opening in experimental cerebral malaria. PLoS Pathog 2012; 8:e1002982; http://dx.doi.org/10.1371/journal.ppat.1002982; PMID: 23133375
- Muldoon LL, Alvarez JI, Begley DJ, Boado RJ, Del Zoppo GJ, Doolittle ND, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab 2013; 33:13 - 21; http://dx.doi.org/10.1038/jcbfm.2012.153; PMID: 23072749
- Abbott NJ. Astrocyte-endothelial interactions and blood-brain barrier permeability. J Anat 2002; 200:629 - 38; http://dx.doi.org/10.1046/j.1469-7580.2002.00064.x; PMID: 12162730
- Hirase T, Staddon JM, Saitou M, Ando-Akatsuka Y, Itoh M, Furuse M, et al. Occludin as a possible determinant of tight junction permeability in endothelial cells. J Cell Sci 1997; 110:1603 - 13; PMID: 9247194
- Piontek J, Winkler L, Wolburg H, Müller SL, Zuleger N, Piehl C, et al. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J 2008; 22:146 - 58; http://dx.doi.org/10.1096/fj.07-8319com; PMID: 17761522
- Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, et al. Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice. J Cell Biol 2003; 161:653 - 60; http://dx.doi.org/10.1083/jcb.200302070; PMID: 12743111
- Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature 2010; 468:562 - 6; http://dx.doi.org/10.1038/nature09513; PMID: 20944625
- Abbott NJ, Rönnbäck L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 2006; 7:41 - 53; http://dx.doi.org/10.1038/nrn1824; PMID: 16371949
- Gavard J, Patel V, Gutkind JS. Angiopoietin-1 prevents VEGF-induced endothelial permeability by sequestering Src through mDia. Dev Cell 2008; 14:25 - 36; http://dx.doi.org/10.1016/j.devcel.2007.10.019; PMID: 18194650
- Finney CA, Hawkes CA, Kain DC, Dhabangi A, Musoke C, Cserti-Gazdewich C, et al. S1P is associated with protection in human and experimental cerebral malaria. Mol Med 2011; 17:717 - 25; http://dx.doi.org/10.2119/molmed.2010.00214; PMID: 21556483
- London NR, Zhu W, Bozza FA, Smith MC, Greif DM, Sorensen LK, et al. Targeting Robo4-dependent Slit signaling to survive the cytokine storm in sepsis and influenza. Sci Transl Med 2010; 2:23ra19; http://dx.doi.org/10.1126/scitranslmed.3000678; PMID: 20375003
- Serghides L, Kim H, Lu Z, Kain DC, Miller C, Francis RC, et al. Inhaled nitric oxide reduces endothelial activation and parasite accumulation in the brain, and enhances survival in experimental cerebral malaria. PLoS One 2011; 6:e27714; http://dx.doi.org/10.1371/journal.pone.0027714; PMID: 22110737
- Erdman LK, Dhabangi A, Musoke C, Conroy AL, Hawkes M, Higgins S, et al. Combinations of host biomarkers predict mortality among Ugandan children with severe malaria: a retrospective case-control study. PLoS One 2011; 6:e17440; http://dx.doi.org/10.1371/journal.pone.0017440; PMID: 21364762
- Conroy AL, Phiri H, Hawkes M, Glover S, Mallewa M, Seydel KB, et al. Endothelium-based biomarkers are associated with cerebral malaria in Malawian children: a retrospective case-control study. PLoS One 2010; 5:e15291; http://dx.doi.org/10.1371/journal.pone.0015291; PMID: 21209923
- Conroy AL, Lafferty EI, Lovegrove FE, Krudsood S, Tangpukdee N, Liles WC, et al. Whole blood angiopoietin-1 and -2 levels discriminate cerebral and severe (non-cerebral) malaria from uncomplicated malaria. Malar J 2009; 8:295; http://dx.doi.org/10.1186/1475-2875-8-295; PMID: 20003529
- Jain V, Lucchi NW, Wilson NO, Blackstock AJ, Nagpal AC, Joel PK, et al. Plasma levels of angiopoietin-1 and -2 predict cerebral malaria outcome in Central India. Malar J 2011; 10:383; http://dx.doi.org/10.1186/1475-2875-10-383; PMID: 22192385
- Lovegrove FE, Tangpukdee N, Opoka RO, Lafferty EI, Rajwans N, Hawkes M, et al. Serum angiopoietin-1 and -2 levels discriminate cerebral malaria from uncomplicated malaria and predict clinical outcome in African children. PLoS One 2009; 4:e4912; http://dx.doi.org/10.1371/journal.pone.0004912; PMID: 19300530
- Conroy AL, Glover SJ, Hawkes M, Erdman LK, Seydel KB, Taylor TE, et al. Angiopoietin-2 levels are associated with retinopathy and predict mortality in Malawian children with cerebral malaria: a retrospective case-control study*. Crit Care Med 2012; 40:952 - 9; http://dx.doi.org/10.1097/CCM.0b013e3182373157; PMID: 22343839
- Rondaij MG, Bierings R, Kragt A, van Mourik JA, Voorberg J. Dynamics and plasticity of Weibel-Palade bodies in endothelial cells. Arterioscler Thromb Vasc Biol 2006; 26:1002 - 7; http://dx.doi.org/10.1161/01.ATV.0000209501.56852.6c; PMID: 16469951
- Fiedler U, Reiss Y, Scharpfenecker M, Grunow V, Koidl S, Thurston G, et al. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med 2006; 12:235 - 9; http://dx.doi.org/10.1038/nm1351; PMID: 16462802
- Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, Piera K, et al. Angiopoietin-2 is associated with decreased endothelial nitric oxide and poor clinical outcome in severe falciparum malaria. Proc Natl Acad Sci U S A 2008; 105:17097 - 102; http://dx.doi.org/10.1073/pnas.0805782105; PMID: 18957536
- Conroy ALS, Zhong KL, Rennie K, Ward M, Sarma P, Molyneux JV, et al. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell Host Microbe 2013; 13:215 - 26; http://dx.doi.org/10.1016/j.chom.2013.01.010; PMID: 23414761
- Prapansilp P, Medana I, Mai NT, Day NP, Phu NH, Yeo TW, et al. A clinicopathological correlation of the expression of the angiopoietin-Tie-2 receptor pathway in the brain of adults with Plasmodium falciparum malaria. Malar J 2013; 12:50; http://dx.doi.org/10.1186/1475-2875-12-50; PMID: 23383853
- Pieh C, Agostini H, Buschbeck C, Krüger M, Schulte-Mönting J, Zirrgiebel U, et al. VEGF-A, VEGFR-1, VEGFR-2 and Tie2 levels in plasma of premature infants: relationship to retinopathy of prematurity. Br J Ophthalmol 2008; 92:689 - 93; http://dx.doi.org/10.1136/bjo.2007.128371; PMID: 18408080
- Steinle JJ, Sharma S, Chin VC. Normal aging involves altered expression of growth factors in the rat choroid. J Gerontol A Biol Sci Med Sci 2008; 63:135 - 40; http://dx.doi.org/10.1093/gerona/63.2.135; PMID: 18314447
- Matsushita K, Morrell CN, Cambien B, Yang SX, Yamakuchi M, Bao C, et al. Nitric oxide regulates exocytosis by S-nitrosylation of N-ethylmaleimide-sensitive factor. Cell 2003; 115:139 - 50; http://dx.doi.org/10.1016/S0092-8674(03)00803-1; PMID: 14567912
- Yeo TW, Lampah DA, Tjitra E, Gitawati R, Kenangalem E, Piera K, et al. Relationship of cell-free hemoglobin to impaired endothelial nitric oxide bioavailability and perfusion in severe falciparum malaria. J Infect Dis 2009; 200:1522 - 9; http://dx.doi.org/10.1086/644641; PMID: 19803726
- Gramaglia I, Sobolewski P, Meays D, Contreras R, Nolan JP, Frangos JA, et al. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat Med 2006; 12:1417 - 22; http://dx.doi.org/10.1038/nm1499; PMID: 17099710
- Yeo TW, Lampah DA, Gitawati R, Tjitra E, Kenangalem E, McNeil YR, et al. Impaired nitric oxide bioavailability and L-arginine reversible endothelial dysfunction in adults with falciparum malaria. J Exp Med 2007; 204:2693 - 704; http://dx.doi.org/10.1084/jem.20070819; PMID: 17954570
- Serirom S, Raharjo WH, Chotivanich K, Loareesuwan S, Kubes P, Ho M. Anti-adhesive effect of nitric oxide on Plasmodium falciparum cytoadherence under flow. Am J Pathol 2003; 162:1651 - 60; http://dx.doi.org/10.1016/S0002-9440(10)64299-X; PMID: 12707049
- Smith AR, Visioli F, Frei B, Hagen TM. Age-related changes in endothelial nitric oxide synthase phosphorylation and nitric oxide dependent vasodilation: evidence for a novel mechanism involving sphingomyelinase and ceramide-activated phosphatase 2A. Aging Cell 2006; 5:391 - 400; http://dx.doi.org/10.1111/j.1474-9726.2006.00232.x; PMID: 16930126
- Lee JF, Zeng Q, Ozaki H, Wang L, Hand AR, Hla T, et al. Dual roles of tight junction-associated protein, zonula occludens-1, in sphingosine 1-phosphate-mediated endothelial chemotaxis and barrier integrity. J Biol Chem 2006; 281:29190 - 200; http://dx.doi.org/10.1074/jbc.M604310200; PMID: 16891661
- Coelho RP, Saini HS, Sato-Bigbee C. Sphingosine-1-phosphate and oligodendrocytes: from cell development to the treatment of multiple sclerosis. Prostaglandins Other Lipid Mediat 2010; 91:139 - 44; http://dx.doi.org/10.1016/j.prostaglandins.2009.04.002; PMID: 19808013
- Brose K, Bland KS, Wang KH, Arnott D, Henzel W, Goodman CS, et al. Slit proteins bind Robo receptors and have an evolutionarily conserved role in repulsive axon guidance. Cell 1999; 96:795 - 806; http://dx.doi.org/10.1016/S0092-8674(00)80590-5; PMID: 10102268
- Stiles J, Jernigan TL. The basics of brain development. Neuropsychol Rev 2010; 20:327 - 48; http://dx.doi.org/10.1007/s11065-010-9148-4; PMID: 21042938
- Saunders NR, Liddelow SA, Dziegielewska KM. Barrier mechanisms in the developing brain. Front Pharmacol 2012; 3:46; http://dx.doi.org/10.3389/fphar.2012.00046; PMID: 22479246
- Reiss AL, Abrams MT, Singer HS, Ross JL, Denckla MB. Brain development, gender and IQ in children. A volumetric imaging study. Brain 1996; 119:1763 - 74; http://dx.doi.org/10.1093/brain/119.5.1763; PMID: 8931596
- Rice D, Barone S Jr.. Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models. Environ Health Perspect 2000; 108:Suppl 3 511 - 33; PMID: 10852851
- Gupta RK, Kanungo M. Glial molecular alterations with mouse brain development and aging: up-regulation of the Kir4.1 and aquaporin-4. Age (Dordr) 2013; 35:59 - 67; http://dx.doi.org/10.1007/s11357-011-9330-5; PMID: 22057895
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist 2005; 11:400 - 7; http://dx.doi.org/10.1177/1073858405278321; PMID: 16151042
- Birbeck GL, Molyneux ME, Kaplan PW, Seydel KB, Chimalizeni YF, Kawaza K, et al. Blantyre Malaria Project Epilepsy Study (BMPES) of neurological outcomes in retinopathy-positive paediatric cerebral malaria survivors: a prospective cohort study. Lancet Neurol 2010; 9:1173 - 81; http://dx.doi.org/10.1016/S1474-4422(10)70270-2; PMID: 21056005
- Boivin MJ, Gladstone MJ, Vokhiwa M, Birbeck GL, Magen JG, Page C, et al. Developmental outcomes in Malawian children with retinopathy-positive cerebral malaria. Trop Med Int Health 2011; 16:263 - 71; http://dx.doi.org/10.1111/j.1365-3156.2010.02704.x; PMID: 21143354
- Zecevic N, Bourgeois JP, Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res 1989; 50:11 - 32; http://dx.doi.org/10.1016/0165-3806(89)90124-7; PMID: 2582602
- Innocenti GM, Price DJ. Exuberance in the development of cortical networks. Nat Rev Neurosci 2005; 6:955 - 65; http://dx.doi.org/10.1038/nrn1790; PMID: 16288299
- Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol 1997; 387:167 - 78; http://dx.doi.org/10.1002/(SICI)1096-9861(19971020)387:2<167::AID-CNE1>3.0.CO;2-Z; PMID: 9336221
- McDonald CR, Elphinstone RE, Kain KC. The impact of placental malaria on neurodevelopment of exposed infants: a role for the complement system?. Trends Parasitol 2013; 29:213 - 9; http://dx.doi.org/10.1016/j.pt.2013.03.005; PMID: 23562777
- Chapman SB, Max JE, Gamino JF, McGlothlin JH, Cliff SN. Discourse plasticity in children after stroke: age at injury and lesion effects. Pediatr Neurol 2003; 29:34 - 41; http://dx.doi.org/10.1016/S0887-8994(03)00012-2; PMID: 13679119
- Durkin MS, Olsen S, Barlow B, Virella A, Connolly ES Jr.. The epidemiology of urban pediatric neurological trauma: evaluation of, and implications for, injury prevention programs. Neurosurgery 1998; 42:300 - 10; http://dx.doi.org/10.1097/00006123-199802000-00052; PMID: 9482180
- Fernández-López D, Faustino J, Daneman R, Zhou L, Lee SY, Derugin N, et al. Blood-brain barrier permeability is increased after acute adult stroke but not neonatal stroke in the rat. J Neurosci 2012; 32:9588 - 600; http://dx.doi.org/10.1523/JNEUROSCI.5977-11.2012; PMID: 22787045
- Claus CP, Tsuru-Aoyagi K, Adwanikar H, Walker B, Manvelyan H, Whetstone W, et al. Age is a determinant of leukocyte infiltration and loss of cortical volume after traumatic brain injury. Dev Neurosci 2010; 32:454 - 65; PMID: 20847543
- Holtzman D, DeVries C, Nguyen H, Olson J, Bensch K. Maturation of resistance to lead encephalopathy: cellular and subcellular mechanisms. Neurotoxicology 1984; 5:97 - 124; PMID: 6542983
- Lawson LJ, Perry VH. The unique characteristics of inflammatory responses in mouse brain are acquired during postnatal development. Eur J Neurosci 1995; 7:1584 - 95; http://dx.doi.org/10.1111/j.1460-9568.1995.tb01154.x; PMID: 7551185
- Anthony DC, Bolton SJ, Fearn S, Perry VH. Age-related effects of interleukin-1 beta on polymorphonuclear neutrophil-dependent increases in blood-brain barrier permeability in rats. Brain 1997; 120:435 - 44; http://dx.doi.org/10.1093/brain/120.3.435; PMID: 9126055
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, et al. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood-brain barrier breakdown. Curr Biol 1998; 8:923 - 6; http://dx.doi.org/10.1016/S0960-9822(07)00373-9; PMID: 9707404
- Stolp HB, Dziegielewska KM, Ek CJ, Habgood MD, Lane MA, Potter AM, et al. Breakdown of the blood-brain barrier to proteins in white matter of the developing brain following systemic inflammation. Cell Tissue Res 2005; 320:369 - 78; http://dx.doi.org/10.1007/s00441-005-1088-6; PMID: 15846513
- van der Heyde HC, Nolan J, Combes V, Gramaglia I, Grau GE. A unified hypothesis for the genesis of cerebral malaria: sequestration, inflammation and hemostasis leading to microcirculatory dysfunction. Trends Parasitol 2006; 22:503 - 8; http://dx.doi.org/10.1016/j.pt.2006.09.002; PMID: 16979941
- Mita-Mendoza NK, van de Hoef DL, Lopera-Mesa TM, Doumbia S, Konate D, Doumbouya M, et al. A potential role for plasma uric acid in the endothelial pathology of Plasmodium falciparum malaria. PLoS One 2013; 8:e54481; http://dx.doi.org/10.1371/journal.pone.0054481; PMID: 23349902
- Gillrie MR, Lee K, Gowda DC, Davis SP, Monestier M, Cui L, et al. Plasmodium falciparum histones induce endothelial proinflammatory response and barrier dysfunction. Am J Pathol 2012; 180:1028 - 39; http://dx.doi.org/10.1016/j.ajpath.2011.11.037; PMID: 22260922
- Lacerda-Queiroz N, Rodrigues DH, Vilela MC, Rachid MA, Soriani FM, Sousa LP, et al. Platelet-activating factor receptor is essential for the development of experimental cerebral malaria. Am J Pathol 2012; 180:246 - 55; http://dx.doi.org/10.1016/j.ajpath.2011.09.038; PMID: 22079430
- Zougbédé S, Miller F, Ravassard P, Rebollo A, Cicéron L, Couraud PO, et al. Metabolic acidosis induced by Plasmodium falciparum intraerythrocytic stages alters blood-brain barrier integrity. J Cereb Blood Flow Metab 2011; 31:514 - 26; http://dx.doi.org/10.1038/jcbfm.2010.121; PMID: 20683453
- Souza MC, Paixão FH, Ferraris FK, Ribeiro I, Henriques Md. Artesunate Exerts a Direct Effect on Endothelial Cell Activation and NF-κB Translocation in a Mechanism Independent of Plasmodium Killing. Malar Res Treat 2012; 2012:679090; http://dx.doi.org/10.1155/2012/679090; PMID: 23097741
- Gray C, McCormick C, Turner G, Craig A. ICAM-1 can play a major role in mediating P. falciparum adhesion to endothelium under flow. Mol Biochem Parasitol 2003; 128:187 - 93; http://dx.doi.org/10.1016/S0166-6851(03)00075-6; PMID: 12742585
- Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med 2004; 10:143 - 5; http://dx.doi.org/10.1038/nm986; PMID: 14745442
- Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, et al. The neuropathology of fatal cerebral malaria in malawian children. Am J Pathol 2011; 178:2146 - 58; http://dx.doi.org/10.1016/j.ajpath.2011.01.016; PMID: 21514429
- Lee WH, Sonntag WE, Lee YW. Aging attenuates radiation-induced expression of pro-inflammatory mediators in rat brain. Neurosci Lett 2010; 476:89 - 93; http://dx.doi.org/10.1016/j.neulet.2010.04.009; PMID: 20385203
- Hobden JA, Masinick SA, Barrett RP, Hazlett LD. Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci 1995; 36:1107 - 14; PMID: 7730020
- Xu YZ, Nygård M, Kristensson K, Bentivoglio M. Regulation of cytokine signaling and T-cell recruitment in the aging mouse brain in response to central inflammatory challenge. Brain Behav Immun 2010; 24:138 - 52; http://dx.doi.org/10.1016/j.bbi.2009.09.006; PMID: 19765643
- Reis PA, Estato V, da Silva TI, d’Avila JC, Siqueira LD, Assis EF, et al. Statins decrease neuroinflammation and prevent cognitive impairment after cerebral malaria. PLoS Pathog 2012; 8:e1003099; http://dx.doi.org/10.1371/journal.ppat.1003099; PMID: 23300448
- Elias RM, Correa-Costa M, Barreto CR, Silva RC, Hayashida CY, Castoldi A, et al. Oxidative stress and modification of renal vascular permeability are associated with acute kidney injury during P. berghei ANKA infection. PLoS One 2012; 7:e44004; http://dx.doi.org/10.1371/journal.pone.0044004; PMID: 22952850
- Lacerda-Queiroz N, Lima OC, Carneiro CM, Vilela MC, Teixeira AL, Teixeira-Carvalho A, et al. Plasmodium berghei NK65 induces cerebral leukocyte recruitment in vivo: an intravital microscopic study. Acta Trop 2011; 120:31 - 9; http://dx.doi.org/10.1016/j.actatropica.2011.04.020; PMID: 21722620
- Lacerda-Queiroz N, Rachid MA, Teixeira MM, Teixeira AL. The role of platelet-activating factor receptor (PAFR) in lung pathology during experimental malaria. Int J Parasitol 2013; 43:11 - 5; http://dx.doi.org/10.1016/j.ijpara.2012.11.008; PMID: 23260771
- Epiphanio S, Campos MG, Pamplona A, Carapau D, Pena AC, Ataíde R, et al. VEGF promotes malaria-associated acute lung injury in mice. PLoS Pathog 2010; 6:e1000916; http://dx.doi.org/10.1371/journal.ppat.1000916; PMID: 20502682
- Susomboon P, Maneerat Y, Dekumyoy P, Kalambaheti T, Iwagami M, Komaki-Yasuda K, et al. Down-regulation of tight junction mRNAs in human endothelial cells co-cultured with Plasmodium falciparum-infected erythrocytes. Parasitol Int 2006; 55:107 - 12; http://dx.doi.org/10.1016/j.parint.2005.11.054; PMID: 16388977
- Tripathi AK, Sullivan DJ, Stins MF. Plasmodium falciparum-infected erythrocytes decrease the integrity of human blood-brain barrier endothelial cell monolayers. J Infect Dis 2007; 195:942 - 50; http://dx.doi.org/10.1086/512083; PMID: 17330783
- Brown H, Rogerson S, Taylor T, Tembo M, Mwenechanya J, Molyneux M, et al. Blood-brain barrier function in cerebral malaria in Malawian children. Am J Trop Med Hyg 2001; 64:207 - 13; PMID: 11442219
- Brown H, Turner G, Rogerson S, Tembo M, Mwenechanya J, Molyneux M, et al. Cytokine expression in the brain in human cerebral malaria. J Infect Dis 1999; 180:1742 - 6; http://dx.doi.org/10.1086/315078; PMID: 10515846
- Mooradian AD, Haas MJ, Chehade JM. Age-related changes in rat cerebral occludin and zonula occludens-1 (ZO-1). Mech Ageing Dev 2003; 124:143 - 6; http://dx.doi.org/10.1016/S0047-6374(02)00041-6; PMID: 12633933
- D’Alessandro S, Basilico N, Prato M. Effects of Plasmodium falciparum-infected erythrocytes on matrix metalloproteinase-9 regulation in human microvascular endothelial cells. Asian Pac J Trop Med 2013; 6:195 - 9; http://dx.doi.org/10.1016/S1995-7645(13)60022-X; PMID: 23375032
- Chen F, Ohashi N, Li W, Eckman C, Nguyen JH. Disruptions of occludin and claudin-5 in brain endothelial cells in vitro and in brains of mice with acute liver failure. Hepatology 2009; 50:1914 - 23; http://dx.doi.org/10.1002/hep.23203; PMID: 19821483
- Yu TY, Pang JH, Wu KP, Chen MJ, Chen CH, Tsai WC. Aging is associated with increased activities of matrix metalloproteinase-2 and -9 in tenocytes. BMC Musculoskelet Disord 2013; 14:2; http://dx.doi.org/10.1186/1471-2474-14-2; PMID: 23281803
- Pamplona A, Ferreira A, Balla J, Jeney V, Balla G, Epiphanio S, et al. Heme oxygenase-1 and carbon monoxide suppress the pathogenesis of experimental cerebral malaria. Nat Med 2007; 13:703 - 10; http://dx.doi.org/10.1038/nm1586; PMID: 17496899
- Seixas E, Gozzelino R, Chora A, Ferreira A, Silva G, Larsen R, et al. Heme oxygenase-1 affords protection against noncerebral forms of severe malaria. Proc Natl Acad Sci U S A 2009; 106:15837 - 42; http://dx.doi.org/10.1073/pnas.0903419106; PMID: 19706490
- Lin T, Kwak YH, Sammy F, He P, Thundivalappil S, Sun G, et al. Synergistic inflammation is induced by blood degradation products with microbial Toll-like receptor agonists and is blocked by hemopexin. J Infect Dis 2010; 202:624 - 32; http://dx.doi.org/10.1086/654929; PMID: 20617898
- Balla J, Jacob HS, Balla G, Nath K, Eaton JW, Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci U S A 1993; 90:9285 - 9; http://dx.doi.org/10.1073/pnas.90.20.9285; PMID: 8415693
- Jeney V, Balla J, Yachie A, Varga Z, Vercellotti GM, Eaton JW, et al. Pro-oxidant and cytotoxic effects of circulating heme. Blood 2002; 100:879 - 87; http://dx.doi.org/10.1182/blood.V100.3.879; PMID: 12130498
- Motterlini R, Foresti R, Intaglietta M, Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol 1996; 270:H107 - 14; PMID: 8769740
- Di Giulio C, Verratti V, Artese L, Petruccelli G, Walski M, Pokorski M. Aging and expression of heme oxygenase-1 and endothelin-1 in the rat carotid body after chronic hypoxia. J Physiol Pharmacol 2009; 60:Suppl 5 41 - 4; PMID: 20134037
- Kang MJ, Kim HJ, Kim HK, Lee JY, Kim DH, Jung KJ, et al. The effect of age and calorie restriction on HIF-1-responsive genes in aged liver. Biogerontology 2005; 6:27 - 37; http://dx.doi.org/10.1007/s10522-004-7381-z; PMID: 15834661
- Tripathi AK, Sha W, Shulaev V, Stins MF, Sullivan DJ Jr.. Plasmodium falciparum-infected erythrocytes induce NF-kappaB regulated inflammatory pathways in human cerebral endothelium. Blood 2009; 114:4243 - 52; http://dx.doi.org/10.1182/blood-2009-06-226415; PMID: 19713460
- Pino P, Vouldoukis I, Kolb JP, Mahmoudi N, Desportes-Livage I, Bricaire F, et al. Plasmodium falciparum--infected erythrocyte adhesion induces caspase activation and apoptosis in human endothelial cells. J Infect Dis 2003; 187:1283 - 90; http://dx.doi.org/10.1086/373992; PMID: 12696008
- Taoufiq Z, Pino P, N’dilimabaka N, Arrouss I, Assi S, Soubrier F, et al. Atorvastatin prevents Plasmodium falciparum cytoadherence and endothelial damage. Malar J 2011; 10:52; http://dx.doi.org/10.1186/1475-2875-10-52; PMID: 21356073
- Souraud JB, Briolant S, Dormoi J, Mosnier J, Savini H, Baret E, et al. Atorvastatin treatment is effective when used in combination with mefloquine in an experimental cerebral malaria murine model. Malar J 2012; 11:13; http://dx.doi.org/10.1186/1475-2875-11-13; PMID: 22233563
- Hemmer CJ, Löbermann M, Unverricht M, Vogt A, Krause R, Reisinger EC. Activated protein C protects vascular endothelial cells from apoptosis in malaria and in sepsis. Trop Med Int Health 2011; 16:906 - 13; http://dx.doi.org/10.1111/j.1365-3156.2011.02788.x; PMID: 21615630
- Dondorp A, Nosten F, Stepniewska K, Day N, White N, South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717 - 25; http://dx.doi.org/10.1016/S0140-6736(05)67176-0; PMID: 16125588
- Dondorp AM, Fanello CI, Hendriksen IC, Gomes E, Seni A, Chhaganlal KD, et al, AQUAMAT group. Artesunate versus quinine in the treatment of severe falciparum malaria in African children (AQUAMAT): an open-label, randomised trial. Lancet 2010; 376:1647 - 57; http://dx.doi.org/10.1016/S0140-6736(10)61924-1; PMID: 21062666
- Dondorp AM, Lee SJ, Faiz MA, Mishra S, Price R, Tjitra E, et al. The relationship between age and the manifestations of and mortality associated with severe malaria. Clin Infect Dis 2008; 47:151 - 7; http://dx.doi.org/10.1086/589287; PMID: 18533842
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, Haring D, et al. Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 2012; 379:413 - 31; http://dx.doi.org/10.1016/S0140-6736(12)60034-8; PMID: 22305225
- Kampondeni SD, Potchen MJ, Beare NA, Seydel KB, Glover SJ, Taylor TE, et al. MRI findings in a cohort of brain injured survivors of pediatric cerebral malaria. Am J Trop Med Hyg 2013; 88:542 - 6; http://dx.doi.org/10.4269/ajtmh.12-0538; PMID: 23339204
- Boivin MJ. Effects of early cerebral malaria on cognitive ability in Senegalese children. J Dev Behav Pediatr 2002; 23:353 - 64; http://dx.doi.org/10.1097/00004703-200210000-00010; PMID: 12394524
- Carter JA, Neville BG, Newton CR. Neuro-cognitive impairment following acquired central nervous system infections in childhood: a systematic review. Brain Res Brain Res Rev 2003; 43:57 - 69; http://dx.doi.org/10.1016/S0165-0173(03)00192-9; PMID: 14499462
- Carter JA, Ross AJ, Neville BG, Obiero E, Katana K, Mung’ala-Odera V, et al. Developmental impairments following severe falciparum malaria in children. Trop Med Int Health 2005; 10:3 - 10; http://dx.doi.org/10.1111/j.1365-3156.2004.01345.x; PMID: 15655008
- Carter JA, Mung’ala-Odera V, Neville BG, Murira G, Mturi N, Musumba C, et al. Persistent neurocognitive impairments associated with severe falciparum malaria in Kenyan children. J Neurol Neurosurg Psychiatry 2005; 76:476 - 81; http://dx.doi.org/10.1136/jnnp.2004.043893; PMID: 15774431
- Boivin MJ, Bangirana P, Byarugaba J, Opoka RO, Idro R, Jurek AM, et al. Cognitive impairment after cerebral malaria in children: a prospective study. Pediatrics 2007; 119:e360 - 6; http://dx.doi.org/10.1542/peds.2006-2027; PMID: 17224457
- Beare NA, Southern C, Kayira K, Taylor TE, Harding SP. Visual outcomes in children in Malawi following retinopathy of severe malaria. Br J Ophthalmol 2004; 88:321 - 4; http://dx.doi.org/10.1136/bjo.2003.025924; PMID: 14977760
- Haslett P. Retinal haemorrhages in Zambian children with cerebral malaria. J Trop Pediatr 1991; 37:86 - 7; http://dx.doi.org/10.1093/tropej/37.2.86; PMID: 2027172
- Kayembe D, Maertens K, De Laey JJ. [Ocular complications of cerebral malaria]. Bull Soc Belge Ophtalmol 1980; 190:53 - 60; PMID: 7284663
- Molyneux ME, Taylor TE, Wirima JJ, Borgstein A. Clinical features and prognostic indicators in paediatric cerebral malaria: a study of 131 comatose Malawian children. Q J Med 1989; 71:441 - 59; PMID: 2690177
- Hero M, Harding SP, Riva CE, Winstanley PA, Peshu N, Marsh K. Photographic and angiographic characterization of the retina of Kenyan children with severe malaria. Arch Ophthalmol 1997; 115:997 - 1003; http://dx.doi.org/10.1001/archopht.1997.01100160167005; PMID: 9258221
- Essuman VA, Ntim-Amponsah CT, Astrup BS, Adjei GO, Kurtzhals JA, Ndanu TA, et al. Retinopathy in severe malaria in Ghanaian children--overlap between fundus changes in cerebral and non-cerebral malaria. Malar J 2010; 9:232; http://dx.doi.org/10.1186/1475-2875-9-232; PMID: 20704742
- Biswas J, Fogla R, Srinivasan P, Narayan S, Haranath K, Badrinath V. Ocular malaria. A clinical and histopathologic study. Ophthalmology 1996; 103:1471 - 5; PMID: 8841308
- Hidayat AA, Nalbandian RM, Sammons DW, Fleischman JA, Johnson TE. The diagnostic histopathologic features of ocular malaria. Ophthalmology 1993; 100:1183 - 6; PMID: 8341499
- Mehta SA, Ansari AS, Jiandani P. Ophthalmoscopic findings in adult patients with severe falciparum malaria. Ocul Immunol Inflamm 2008; 16:239 - 41; http://dx.doi.org/10.1080/09273940802409977; PMID: 19065421
- Kochar DK, Shubhakaran, Kumawat BL, Thanvi I, Joshi A, Vyas SP. Ophthalmoscopic abnormalities in adults with falciparum malaria. QJM 1998; 91:845 - 52; http://dx.doi.org/10.1093/qjmed/91.12.845; PMID: 10024950
- Abu Sayeed A, Maude RJ, Hasan MU, Mohammed N, Hoque MG, Dondorp AM, et al. Malarial retinopathy in Bangladeshi adults. Am J Trop Med Hyg 2011; 84:141 - 7; http://dx.doi.org/10.4269/ajtmh.2011.10-0205; PMID: 21212217
- Maude RJ, Beare NA, Abu Sayeed A, Chang CC, Charunwatthana P, Faiz MA, et al. The spectrum of retinopathy in adults with Plasmodium falciparum malaria. Trans R Soc Trop Med Hyg 2009; 103:665 - 71; http://dx.doi.org/10.1016/j.trstmh.2009.03.001; PMID: 19344925
- Beare NA, Southern C, Chalira C, Taylor TE, Molyneux ME, Harding SP. Prognostic significance and course of retinopathy in children with severe malaria. Arch Ophthalmol 2004; 122:1141 - 7; http://dx.doi.org/10.1001/archopht.122.8.1141; PMID: 15302654
- Hirneiss C, Klauss V, Wilke M, Kampik A, Taylor T, Lewallen S. [Ocular changes in tropical malaria with cerebral involvement--results from the Blantyre Malaria Project]. Klin Monbl Augenheilkd 2005; 222:704 - 8; PMID: 16175479
- White VA, Lewallen S, Beare N, Kayira K, Carr RA, Taylor TE. Correlation of retinal haemorrhages with brain haemorrhages in children dying of cerebral malaria in Malawi. Trans R Soc Trop Med Hyg 2001; 95:618 - 21; http://dx.doi.org/10.1016/S0035-9203(01)90097-5; PMID: 11816433
- Lewallen S, White VA, Whitten RO, Gardiner J, Hoar B, Lindley J, et al. Clinical-histopathological correlation of the abnormal retinal vessels in cerebral malaria. Arch Ophthalmol 2000; 118:924 - 8; PMID: 10900105
- Beare NA, Harding SP, Taylor TE, Lewallen S, Molyneux ME. Perfusion abnormalities in children with cerebral malaria and malarial retinopathy. J Infect Dis 2009; 199:263 - 71; http://dx.doi.org/10.1086/595735; PMID: 18999956
- Beare NA, Lewallen S, Taylor TE, Molyneux ME. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol 2011; 6:349 - 55; http://dx.doi.org/10.2217/fmb.11.3; PMID: 21449844
- Medana IM, Day NP, Hien TT, Mai NT, Bethell D, Phu NH, et al. Axonal injury in cerebral malaria. Am J Pathol 2002; 160:655 - 66; http://dx.doi.org/10.1016/S0002-9440(10)64885-7; PMID: 11839586
- Medana IM, Turner GD. Human cerebral malaria and the blood-brain barrier. Int J Parasitol 2006; 36:555 - 68; http://dx.doi.org/10.1016/j.ijpara.2006.02.004; PMID: 16616145
- Patnaik JK, Das BS, Mishra SK, Mohanty S, Satpathy SK, Mohanty D. Vascular clogging, mononuclear cell margination, and enhanced vascular permeability in the pathogenesis of human cerebral malaria. Am J Trop Med Hyg 1994; 51:642 - 7; PMID: 7985757
- Oo MM, Aikawa M, Than T, Aye TM, Myint PT, Igarashi I, et al. Human cerebral malaria: a pathological study. J Neuropathol Exp Neurol 1987; 46:223 - 31; http://dx.doi.org/10.1097/00005072-198703000-00009; PMID: 3546601
- Anstrom JA, Brown WR, Moody DM, Thore CR, Challa VR, Block SM. Anatomical analysis of the developing cerebral vasculature in premature neonates: absence of precapillary arteriole-to-venous shunts. Pediatr Res 2002; 52:554 - 60; http://dx.doi.org/10.1203/00006450-200210000-00015; PMID: 12357050
- Ballabh P, Braun A, Nedergaard M. The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 2004; 16:1 - 13; http://dx.doi.org/10.1016/j.nbd.2003.12.016; PMID: 15207256
- Namutangula B, Ndeezi G, Byarugaba JS, Tumwine JK. Mannitol as adjunct therapy for childhood cerebral malaria in Uganda: a randomized clinical trial. Malar J 2007; 6:138; http://dx.doi.org/10.1186/1475-2875-6-138; PMID: 17958887
- Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, et al. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N Engl J Med 1982; 306:313 - 9; http://dx.doi.org/10.1056/NEJM198202113060601; PMID: 7033788
- Hoffman SL, Rustama D, Punjabi NH, Surampaet B, Sanjaya B, Dimpudus AJ, et al. High-dose dexamethasone in quinine-treated patients with cerebral malaria: a double-blind, placebo-controlled trial. J Infect Dis 1988; 158:325 - 31; http://dx.doi.org/10.1093/infdis/158.2.325; PMID: 3042874
- Mohanty S, Mishra SK, Patnaik R, Dutt AK, Pradhan S, Das B, et al. Brain swelling and mannitol therapy in adult cerebral malaria: a randomized trial. Clin Infect Dis 2011; 53:349 - 55; http://dx.doi.org/10.1093/cid/cir405; PMID: 21810747
- Newton CR, Kirkham FJ, Winstanley PA, Pasvol G, Peshu N, Warrell DA, et al. Intracranial pressure in African children with cerebral malaria. Lancet 1991; 337:573 - 6; http://dx.doi.org/10.1016/0140-6736(91)91638-B; PMID: 1671941
- Waller D, Crawley J, Nosten F, Chapman D, Krishna S, Craddock C, et al. Intracranial pressure in childhood cerebral malaria. Trans R Soc Trop Med Hyg 1991; 85:362 - 4; http://dx.doi.org/10.1016/0035-9203(91)90291-6; PMID: 1949139
- Lewallen S, Taylor TE, Molyneux ME, Wills BA, Courtright P. Ocular fundus findings in Malawian children with cerebral malaria. Ophthalmology 1993; 100:857 - 61; PMID: 8510897
- Potchen MJ, Kampondeni SD, Seydel KB, Birbeck GL, Hammond CA, Bradley WG, et al. Acute brain MRI findings in 120 Malawian children with cerebral malaria: new insights into an ancient disease. AJNR Am J Neuroradiol 2012; 33:1740 - 6; http://dx.doi.org/10.3174/ajnr.A3035; PMID: 22517285
- Looareesuwan S, Wilairatana P, Krishna S, Kendall B, Vannaphan S, Viravan C, et al. Magnetic resonance imaging of the brain in patients with cerebral malaria. Clin Infect Dis 1995; 21:300 - 9; http://dx.doi.org/10.1093/clinids/21.2.300; PMID: 8562735
- Medana IM, Day NP, Sachanonta N, Mai NT, Dondorp AM, Pongponratn E, et al. Coma in fatal adult human malaria is not caused by cerebral oedema. Malar J 2011; 10:267; http://dx.doi.org/10.1186/1475-2875-10-267; PMID: 21923924
- Okoromah CA, Afolabi BB, Wall EC. Mannitol and other osmotic diuretics as adjuncts for treating cerebral malaria. Cochrane Database Syst Rev 2011; CD004615; PMID: 21491391
- Hawkes M, Opoka RO, Namasopo S, Miller C, Thorpe KE, Lavery JV, et al. Inhaled nitric oxide for the adjunctive therapy of severe malaria: protocol for a randomized controlled trial. Trials 2011; 12:176; http://dx.doi.org/10.1186/1745-6215-12-176; PMID: 21752262
- Hawkes M, Opoka RO, Namasopo S, Miller C, Conroy AL, Serghides L, et al. Nitric oxide for the adjunctive treatment of severe malaria: hypothesis and rationale. Med Hypotheses 2011; 77:437 - 44; http://dx.doi.org/10.1016/j.mehy.2011.06.003; PMID: 21745716
- Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med 2005; 353:23 - 32; http://dx.doi.org/10.1056/NEJMoa043514; PMID: 16000353