Abstract
Streptococcus suis (S. suis) is a family of pathogenic gram-positive bacterial strains that represents a primary health problem in the swine industry worldwide. S. suis is also an emerging zoonotic pathogen that causes severe human infections clinically featuring with varied diseases/syndromes (such as meningitis, septicemia, and arthritis). Over the past few decades, continued efforts have made significant progress toward better understanding this zoonotic infectious entity, contributing in part to the elucidation of the molecular mechanism underlying its high pathogenicity. This review is aimed at presenting an updated overview of this pathogen from the perspective of molecular epidemiology, clinical diagnosis and typing, virulence mechanism, and protective antigens contributing to its zoonosis.
Keywords: :
Introduction
Streptococcosis is regarded as a leading infectious disease in the swine industry, that clinically features with meningitis, septicemia, or arthritis and annually results in significant economic loss worldwide.Citation1 Streptococcus suis (S. suis) that was initially reported in 1954Citation2 has been demonstrated as an etiological agent for this kind of frequently-occurring bacterial infection.Citation1,Citation3 Indeed, S. suis, a complex population consisting of heterogeneous strains,Citation4 can be classified into 35 serotypes (1–34, 1/2) based on the differentiation of capsule antigens.Citation1,Citation3 Based on the varied virulence of these bacteria, they may be categorized into highly-pathogenic, weakly-pathogenic (hypo-virulent), and nonpathogenic (avirulent) strains.Citation1 Generally, serotype 2 of S. suis (SS2) is considered to be the most virulent, and is frequently isolated from clinically-diseased piglets.Citation1 In fact, serotype 9 of S. suis is also one of the most important serotypes in several countries. Of particular note, SS2 seems to be a previously neglected but recently emerging human pathogen,Citation5 whose infection has become increasingly potent, especially in the southeast Asian countries like Thailand,Citation6 Vietnam,Citation7 and China.Citation8,Citation9
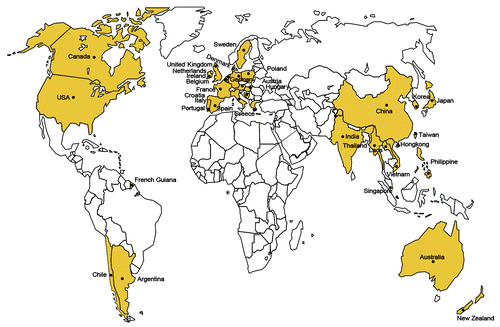
As the primary agent of meningitis, septicemia, arthritis and as an opportunistic pathogen in the case of pneumonia,Citation1,Citation5 S. suis have been reported to have spread over 30 countries and/or regions () and has claimed no less than 1600 human cases, some of which were fatal.Citation2 Also, similar clinical symptoms including bacterial meningitis, septicemia, and arthritis are frequently observed in human SS2 infections.Citation2,Citation3 Occasionally, serotypes other than SS2, including SS1,Citation10 SS4,Citation10 SS5,Citation11,Citation12 SS14,Citation13,Citation14 SS16,Citation15,Citation16 and SS24Citation11 can also be found to function as the causative agents responsible for sporadic cases of human S. suis infection.Citation3 Of note, two big outbreaks of human SS2 endemics which occurred in China, in 1998 and 2005, respectively,Citation9,Citation17,Citation18 have raised serious concerns in public health and have challenged the conventional opinion that human SS2 infections are only present in sporadic cases.Citation2,Citation8,Citation19 Unfortunately, no specific/effective human therapeutics or vaccine against SS2 infections is available thus far. Considering the severity (high mortality and modality) of SS2 infection in humans,Citation5,Citation8 it is important to develop a method for convenient and quick diagnosis, which can be applied toward local SS2 detection.Citation4,Citation18
Figure 1. Global epidemiology of human SS2 infections. Countries/regions with human cases of SS2 infections were labeled and highlighted in yellow. Adapted from references Citation2 and Citation48 with permission.
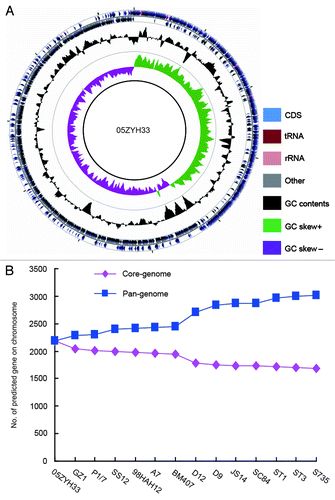
Over the past four decades, significant progress has been made toward better understanding the highly infectious clones of S. suis. At the time of formulating this review, 1104 articles were available in PubMed regarding S. suis (http://www.ncbi.nlm.nih.gov/pubmed/?term=Streptococcus+suis).Totally, over 20 bacterial virulence-associated factors have been identified that include capsular polysaccharides (CPS),Citation20 Muramidase-released protein (MRP),Citation21 and Suilysin (SLY).Citation22 To date, genomic sequences of a collection of S. suis strains are available (), the majority of which are derived from SS2 species,Citation23,Citation24 except two newly-released genomes which correspond to SS3Citation25 and SS14,Citation26 respectively. Genomic mining combined with bacterial genetics have elucidated that Chinese epidemic strains of highly pathogenic S. suis 2 carry a specific 89K PAI (pathogenicity island).Citation23,Citation27 Further studies suggested that 89K PAI with a transposon-like essence can undergo GI-type T4SS-mediated horizontal transfer in epidemic SS2 species.Citation28 The systematic elucidation of the of S. suis pathogenesis in the Omics Era was illustrated by functional definition of a collection of other new genes or putative orthologs (such as Zur, a zinc uptake regulator,Citation29 CovR, an orphan response regulator,Citation30 and Rgg-like transcription factorCitation31) following the release of the genome sequence of SS2 (e.g., 05ZYH33).Citation23 Although we have gained a partial glimpse of the molecular mechanism underlying the high pathogenicity of SS2 itself, we are still lacking further insights into the interface between the SS2 pathogen and the host it infects.Citation3,Citation8
Figure 2. Circular diagram and pan-genome analyses of SS2 genome. (A) Circular diagram of representative SS2 genome. (B) Pan-genome analyses of S. suis 2 species.
In this review, we aim to describe an updated but partial picture of SS2 as an emerging infectious agent, which centers on five aspects: global epidemiology/distribution, clinical diagnostics/typing, pathogenesis, protective antigen/candidate vaccine, and zoonotic potential.
An Overview of S. suis
General microbiology of S. suis
S. suis is a group of heterogeneous gram-positive bacteria that were earlier classified into Lancefield groups R, S, and T ().Citation1 These bacteria are facultative anaerobes with a spherical/ovoid shape which exist in pairs and/or short chains (). Generally, these microorganisms show either α-hemolysis when growing on selective plates of horse blood agarCitation1 (). Given the variation in their CPS antigens, 35 serotypes have been proposed for S. suis population.Citation1 Very few studies of pathogenicity have been done for serotypes other than serotypes 2, 1, and 7.Citation1 Among them, SS2 is recognized as the most virulent species that is frequently associated with diseased pigs and often causes an opportunistic infection of adults having occupational contact with pig carcass or pork-related products.Citation2,Citation5
Figure 3. Characterization of S. suis and its natural host piglets. (A) Colony phenotype of S. suis serotype 2 grown on THB plate with 5% sheep blood. (B) Gram staining analyses of S. suis serotype 2 grown in liquid THB media. It was adapted from reference Citation18 with permission. (C) Scanning electronic microscopic analyses of S. suis serotype 2 collected from overnight culture in THB media. (D) Phenotypic characterization of the reservoir of S. suis serotype 2, piglets maintained in backyard.
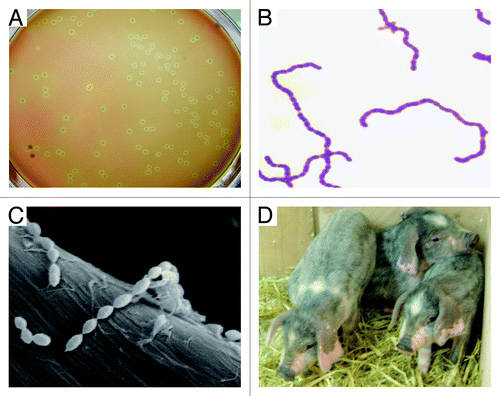
S. suis, an important animal pathogen, naturally inhabits in the upper respiratory (particularly the tonsils and nasal cavities), genital, and alimentary tracts of piglets.Citation1,Citation32 In addition to the natural host swine (), this pathogen has been suggested to be isolated from a wide range of other animals, such as horses, dogs, and cats.Citation1,Citation33 Of note, some variants of S. suis probably have evolved into highly infectious zoonotic agents that can cause meningitis, septicemia, arthritis, and even streptococcal toxic shock-like syndrome (which can cause rapid death) in humans.Citation9,Citation17,Citation18,Citation34 Soon after the big outbreak of human SS2 infections in China, in 2005, serious concerns from both the public health and scientific community have been raised.Citation18 Toward better understanding and prevention/control of SS2 infections, multiple lines of new bacterial virulence determinants (such as salK–salR two component system,Citation27 FeoB transporter,Citation35 and Rgg regulatorCitation31) have been identified, and fast assays using PCR-based molecular detectionCitation18 as well as ELISA (enzyme-linked immunosorbent assay)-guided diagnostics were establishedCitation36 (). Most recently, epidemiological investigations conducted in Vietnam proposed that (1) pig population in slaughterhouses is a major reservoir of SS2 with a capacity to cause human infectionsCitation37 and (2) the most important risk factors of human S. suis infections are consecutively eating “high risk” pork-derived dishes, occupational exposure to pigs and pork-related products, and preparation of pork in the presence of skin lesions.Citation38 The availability of the epidemiological knowledge on human SS2 infections is critical to improve the current situation of public awareness of SS2 infections and to effectively prevent the potential occupational infections caused by SS2.
Table 1. Approaches for detection, identification and typing of Streptococcus suis
Genomics/proteomics-based glimpse of S. suis
Genomics/proteomics approaches to probe S. suis have yielded unprecedented comprehensive information/knowledge about this pathogen.Citation8,Citation39 At the time of writing this review, no less than 14 genomes of S. suis strains have been available in PubMed (http://www.ncbi.nlm.nih.gov/sites/entrez?db=genome&cmd=DetailsSearch&term=streptococcus+suis&save_search=false), most of which were completed by the research groups in China (). Genomic sequence analyses of S. suis showed that (1) all the sequenced genomes feature with the nearly same average GC content, ~41%, indicating slight evolutionary conservation; (2) genome size varies markedly from 1 640 446 nt (05HAS68, NZ_AARD00000000) to 2 146 229 nt (BM407, NC_012926), reflecting the genomic flexibility present in these species (); (3) the number of putative protein-encoding genes is dramatically different (1559 for 05HAS68, and 1932 for BM407), suggesting that nearly 1/5–1/6 of total genes are not essential (redundant) for bacterial viability, and might confer some new/unknown functions to adapt to varied environmental niches. Functional/comparative genomics of S. suis has defined a series of new infection/virulence-related determinants such as ArgR regulator,Citation40 SspA, a subtilisin-like protease,Citation41 CiaRH two-component regulatory system,Citation42 and so on.
On the other hand, proteomics analyses of Chinese epidemic SS2 strain 05ZYH33 identified 373 proteins in total from 834 processed spots.Citation43 Using an immune-proteomic approach, Lu’s group revealed 11 membrane-related proteins from Chinese vaccine strain SS2-HA9801Citation44 and 9 extracellular antigenic proteins from the virulent Chinese SS2 strain ZY05719,Citation45 respectively. Using a similar strategy, Wu and coauthors from the same group addressed two strains of SS9, another prevalent serotype of S. suis (GZ0565 and SH040197), and observed 13 candidate proteins, five of which are virulence-associated factors.Citation46 Additionally, eight immunogenic proteins localized on bacterial surface were determined in strain GZ0565, including extracellular solute-binding protein.Citation47 These findings might provide a solid/reasonable basis for development of subunit vaccine candidates.Citation39
Epidemiology of S. suis Infection
Geographic distribution of human SS2 infections
As a swine pathogen, S. suis was first reported by a vegetarian in 1954,Citation2 while its zoonotic role could be traced to a human SS2 meningitis case in Denmark, in 1968.Citation1 In light of the available literature with human S. suis infection recorded thus far, we expect that S. suis infections have been involved in no less than 30 countries and/or regions (), and resulted in around 1600 cases of severe human infections.Citation2,Citation8,Citation48 In North America (United StatesCitation49,Citation50 and CanadaCitation51,Citation52) and the South American countries (Argentina,Citation53,Citation54 Chile,Citation55 and French GuianaCitation56) only a very few cases of human SS2 infections were reported. Human SS2 infections are featuring with sporadic cases in Europe (Ireland,Citation3 the United Kingdom [UK],Citation3 FranceCitation3,, Spain,Citation57 Netherlands,Citation58 Belgium,Citation3 Poland,Citation61 Sweden,Citation3 Denmark,Citation1 Germany,Citation62 Hungary,Citation3 Austria,Citation63 Croatia,Citation64 ItalyCitation65-Citation67, Greece,Citation68 and PortugalCitation1), some Asian countries (Laos,Citation1 Singapore,Citation33,Citation69 India,Citation2,Citation3,Citation70 Korea,Citation71-Citation73 Japan,Citation74 Hong Kong,Citation75-Citation80 Taiwan,Citation33,Citation81,Citation82 and PhilippineCitation2,Citation3), Australia,Citation83,Citation84 and New Zealand.Citation85,Citation86 So far, endemics of human SS2 infections was only observed in two Asian counties VietnamCitation2,Citation7,Citation16,Citation37,Citation38 and Thailand.Citation6,Citation11,Citation14,Citation87 Of particular note, coexistence of sporadic cases and epidemics of human SS2 infections were present in China.Citation4,Citation8,Citation9,Citation17-Citation19,Citation33,Citation48 It seemed true that the majority of human SS2 infection cases occurred in southeast Asia (especially Vietnam, Thailand, and China), indicating an obvious geographic tropism (). Although we are not quite sure what mechanism can explain such kind of tropism, we anticipate that the following factors are probably correlated with frequent occurrence of human SS2 infections in above countries, which include (1) similar local climates and/or environments; (2) backyard cultivation of pigs; and (3) popular consumption of raw pork sold in the wet market.
Current situation of human S. suis infections in Europe and North America
During the past 40 years, around 100 cases of human S. suis infections were estimated in European countries. Among them, the top three countries in the history of human SS2 infections recorded are Netherlands (41 cases), United Kingdom (15 cases), and Denmark (12 cases).Citation2 The rest of the European countries (such as France and Germany) had less than ten sporadic cases of human infection.Citation2 Moreover, the prevalent type of clinical disease caused by S. suis infections in these countries is bacterial meningitis.
Although human S. suis infections are considered sporadic cases in most countries,Citation2,Citation5,Citation8 it is unbelievable that its zoonotic infectious events are rarely reported in North America (Canada and USA), two huge countries with numerous, large, and frequent swine operations.Citation3,Citation5 To the best of our knowledge, only three human SS2 meningitis cases were confirmed in USA,Citation49,Citation50,Citation89 and three cases were diagnosed in Canada (one case was due to SS14, and the other 2 cases were caused by SS2Citation51,Citation52). Gottschalk and coworkersCitation3 believe that this small number of reported cases from these two huge countries with a big industry for pig cultivation might be attributed to the following two major reasons: (1) Clinical under-diagnosis and/or misdiagnosis of S. suis infection, rather than true absence of this infectious disease, (2) S. suis isolates in North America are less virulent, relative to those from Europe and Asia. A pilot study conducted by Smith et al.Citation90 recently indicated that human infection with S. suis is more common in the United States than what it is generally thought. The reason might lie in underdiagnosis or misdiagnosis, rather than a real lacking of disease.Citation3,Citation91,Citation92 On this issue, we doubt the current situation of S. suis infections as well, though we agree that good hygiene conditions and prevention strategies during the whole process of pork operation (e.g., wearing gloves) might secure the major route of bacterial entry into blood by small cuts in the skins, and greatly decrease the incidents of S. suis infections in North America. Therefore, we believe that it is necessary to employ combined specific approaches (like multiplex PCR plus ELISA) for the re-evaluation of the epidemiological aspects of human S. suis infections in North America. Interestingly, Schmid et al.Citation93 recently demonstrated that American SS2 isolates do not carry 89K PAI, a DNA fragment present in Chinese epidemic strain, which might be helpful for development of an American SS2 strain-specific PCR detection assay.
Situation of human S. suis infections in southeast Asia
Accumulated epidemiological data suggests that over 85% of total cases have occurred in Asian countries.Citation2 The cases of human SS2 infections in the mainland of China and Vietnam are comparable.Citation2,Citation8,Citation16 Relative to the above 2 countries, the human cases clinically infected by S. suis are second.Citation6,Citation14,Citation87 Of being noteworthy, SS2 has been recognized as a pathogen with the mostly-relevance to human bacterial meningitis in southern Vietnam.Citation16 In Hongkong, the specialized administrative region of China, the first human case of S. suis infection was recorded in 1983,Citation78,Citation80 and the accumulated number of S. suis infections were estimated to be about 60 cases.Citation75,Citation78 In particular, two big outbreaks of human SS2 infections in China (1998 and 2005) seriously challenged public health.Citation9,Citation18 In the 1998 epidemic, 14 out of 25 SS2-infected persons died along with an estimated 80 000 pigs.Citation18 In the 2005 epidemic, totally 215 patients had SS2 infections, 38 of which are dead. A similar scenario was also observed when more than 600 pigs were demonstrated to be infected by SS2.Citation9,Citation18 The causative agents of the both epidemics were subsequently determined to be highly invasive clones of strong virulent SS2 strain that seemed to have acquired a new pathogenicity island 89K.Citation8,Citation23,Citation94 Additionally, sporadic human meningitis cases caused by SS2 infections were observed in three other cities of China (Shenzhen City, Chongqing City, and Nanjing City) in 2007,Citation4,Citation95 implying that the situation of SS2 infection in China is complicated. This could be due to variants of S. suis 2 identified in subsequent investigations. It might be of much interest to unveil the possible evolutionary relationship of the Chinese epidemic strain with those of the neighboring country like Vietnam through comparative genomics.
Detection and Typing of S. suis
Fast/effective detection and analyses of S. suis is critical for the prevention and/or diagnosis of endemic S. suis 2 infection in the swine industry as well as for S. suis infected patients. Three types of experimental approaches are available thus far that consist of (1) selective media-based microbiological cultivation, (2) molecular tests, and (3) immunological assays (). Given its advantage in sensitivity and fastness, the second method has been experimentally developed into two subgroups (PCR-based detection plus typing-oriented analyses like pulsed-field gel electrophoresis [PFGE] and restriction fragment length polymorphism [RFLP]) and might be potential in clinical re-confirmation and/or re-valiadtion. Generally, PCR assays can be classified into six kinds among which multiplex-PCR (using sets of specific primers including epf, mrp, and gdh) is appreciably-valid approach to assay SS2 and applied in some countries (). The major four kinds of typing methods include PFGE, RFLP, multi-locus sequence typing (MLST) and random amplified polymorphic DNA (RAPD), some of which have derivatives such as ISR-RFLP (). In general, the three experimental methods (PFGE, RFLP, and RAPD) can return clues about genomic differences between different strains/serotypes. Given direct sequencing of multiple loci (cpn60, dpr, recA, aroA, thrA, gki, and mutS), MLST can directly capture the nucleotide sequence deviation used for typing purpose. Among the immunological approaches, ELISA could be the most popular way to address S. suis infection in some experimental tests (). In fact, different versions of ELISA have been developed that are based on various capture antigens identified to be specifically against S. suis 2. We have also established two kinds ELISA assays (one is based on SAO protein,Citation4,Citation36 the other on Enolase surface antigenCitation96), both of which work well in our trials during field screening and clinical detections. Recently, two more new derivative methods were reported, which are immunochromatographic stripCitation97,Citation98 and electrochemiluminescence immunosensor,Citation99 respectively ().
Molecular Mechanism for Streptococcus suis Infection
Bacterial virulence determinants
The clinical consequence of S. suis infection is determined by the complicated interplay between this zoonotic pathogen and its host. A series of bacterial components as well as a collection of host cell factors contribute to this pathogenesis-related process. So far, almost 60 bacterial components have been identified to be involved in the infection and/or pathogenicity of S. suis (). Of particular note, Wilson and coworkersCitation100 developed a powerful signature-tagged mutagenesis (STM) system for S. suis and identified nearly 20 potential virulence associate elements through screening the library consisting of approximately 2600 mutants (). However, exact roles of these genes needed further verification. According to their general roles in the context of bacterial life cycles, these bacterial virulence-associated factors were temporarily classified into the following three sub-groups (of note some genes probably can be attributed to two different classifications due to their dual characteristics): (1) surface/secreted elements; (2) enzymes/proteases; (3) transcription factors/regulatory systems; and (4) others (transporters/secretion systems) ().
Table 2. Bacterial and host components associated with Streptococcus suis infectivity
Surface/secreted components
For Subgroup 1, a total of 17 genes/gene clusters have been determined thus far to contribute to bacterial pathogenicity (). In addition to six previously identified elements (capsular polysaccharides [CPS],Citation20,Citation101 extracellular protein factor [EF],Citation102 fibronectin binding factor [FBP],Citation103 muramidase-released protein [MRP],Citation102 a protein of 38 kDa localized on bacterial surface [abbreviated 38 kDa],Citation104 and thio-activated hemolysin with known crystal structure [Suilysin, SLY])Citation105,Citation106 plus implication into bacterial meningitis,Citation107 11 more members have been supplemented into this group that include SspA, the surface-associated subtilisin-like serine protease,Citation41,Citation108,Citation109 HtpS, a novel immunogenic cell surface-exposed protein,Citation110 and Sat surface proteinCitation111,Citation112(). Like FBP,Citation103 the gene SSU05_1311 with unknown function was determined to be one more surface anchored fibronectin-binding protein. More importantly, this surface protein functions in vivo in crossing the mucosal epithelia to disseminate, suggesting it is a novel virulence factor.Citation113
Two independent research groups (one from CanadaCitation41 and another from ChinaCitation114) identified the subtilisin-like protease (SspA)-encoding gene (sspA, SSU0757) from the S. suis organism by screening the mutant library and genomic expression library, respectively. SspA proteinase possesses a typical cell wall anchoring signal, LPXTG, at its C-terminus, implying it is a cell surface-displayed protein.Citation108 Infection assays have demonstrated that SspA protein plays critical roles in SS2 pathogenicity.Citation108,Citation109,Citation114 A subsequent study further revealed that S. suis SspA protease might modulate cytokine secretion by macrophages, and thus trigger central nervous system inflammation associated with bacterial meningitis, frequently observed in SS2-infected patients as well as piglets.Citation41
HP0197 is a new a surface protective antigen that was originally identified by Jin’s group in 2009. This immunogenic antigen can elicit obvious humoral antibody response and confer efficient protection against SS2 challenge in the infection models of both mice and pigs.Citation115 Subsequently, HP0197 protein was found to interact with host cell surface glycosaminoglycans (GAGs), and the binding sites were further proved by solving the X-ray structure of N-terminal GAG-binding domain combined with site-directed mutagenesis plus indirect immunofluorescence assay.Citation116 Very recently, the same research group reported that HP0197 is involved in bacterial virulence by evaluating the performance of the isogenic mutant Δhp0197 in both mice and pigs.Citation117 A reasonable interpretation would be that virulence attenuation is due to easier clearance of the Δhp0197 mutant by host immunological system during the stage of infection, and the fast clearance is attributed to the reduced CPS thickness and decreased resistance to phagocytosis.Citation117 Further comparative transcriptomics-based analyses elucidated that expression of CPS synthetic operon is downregulated in the Δhp0197 mutant relative to the wild-type strain.Citation117 Also the introduction of plasmid-borne hp0197 gene into the Δhp0197 mutant can restore the decreased expression level of cps operon to those seen with the wild type strain.Citation117 This observation is consistent with the fact that CcpA, a global carbon catabolite regulator, contributes to bacterial infectivity/pathogenicity.Citation118,Citation119 Preliminary evidence pointed out that HP0197 might determine the Ser-46 phosphorylation level of phospho carrier protein (HPr-46), a partner protein of CcpA in binding the catabolite-responsive elements (cre) of the target operons. Together, it suggested that integration of the posttranslational modification of HPr-46 and CcpA-mediated transcriptional regulation is linked to regulatory network of bacterial virulence.
The serum opacity factor of S. suis (OFS) is a putative member belonging to the family of MSCRAMM (Microbial Surface Components Recognizing Adhesive Matrix Molecules).Citation120 N-terminal region of OFS exhibits similarity to the serum opacity factor of Streptococcus pyogenes and fibronectin-binding protein A (FnBA) of Streptococcus dysgalactiae, and its C-terminus harbors repetitive sequence elements. Crude extract of S. suis and the recombinant OFS protein both possessed serum opacification activity. Experimental infections demonstrated that the deletion of the ofs gene severely impairs S. suis virulence.Citation120
Sao is a surface antigen first identified by Li et al.,Citation121 which can react with convalescent-phase sera from pigs clinically infected by S. suis type 2. Subsequently recombinant Sao formulated with Quil A was found to induce potent opsonizing antibody responses, and confer cross-protection of mice against challenges of virulent heterogeneous S. suis.Citation122 We also discovered that three allelic variants of the sao gene (namely sao-S, sao-M, and sao-L) are present in S. suis population, and we have developed an efficient ELISA method in which Sao-M protein serves as the capture antigen.Citation36 Recent further genetic study suggested that SAO protein is only a minor virulence factorCitation123
Sat (HP272) is another newly-identified surface protein from the S. suis serotype 2,Citation111,Citation112 and two different research groups have shown evidence that recombinant Sat protein can confer effective immuno-protection of mice against SS2 infections, implying that it is a vaccine molecule candidate.Citation111,Citation112 In addition, HtpS, a putative member of histidine triad protein family, was determined to be cell surface-associated protein that was expressed during the infection of Chinese SS2 strain 05ZYH33.Citation110 Moreover, recombinant HtpS protein was demonstrated to function as a protective antigen against SS2 infections.Citation110 Similarly, Zhang et al.Citation124 recently defined another new infection-associated antigen protein, Trag, using in vivo-induced antigen technology (IVIAT). An inactivation of the trag gene was observed to attenuate full virulence of Chinese SS2 strain in the experimental model of Zebrafish.Citation124 As a surface protein, PAPI-2 was found to constitute an ancillary pilus subunit and exhibit ability to confer protection of mice against serious challenge of S. suis.Citation125
Although we have never reported evidence that Zur, a zinc uptake regulator is essential for S. suis virulence,Citation29 Aranda et al. very recently reported that the zinc-binding lipoprotein 103, the structural component of zinc uptake system is associated with the infectivity of Streptococcus suis, implying that zinc uptake system might be involved into bacterial pathogenesis.Citation126 This discrepancy may be attributed to the fact that the deletion of zur only partially affect expression of zinc uptake system genes, whereas inactivation of “103” completely impairs zinc uptake system. That is why the latter could more seriously disrupt the normal zinc metabolism, and in turn lead to virulence attenuation in this sick bacterium.
Enzymes and proteinases
Accumulated data has suggested that no less than 20 bacterial enzymes might be implicated in the manifestation of S. suis virulence (). Among them, eight enzymes are proposed to be virulence factors by Wilson et al.Citation100 using the system of signature-tagged mutagenesis (). Four of them are generally regarded as enzymes of central metabolism, which separately correspond to (1) GlnA, glutamine synthetase,Citation127 (2) Gdh, glutamate dehydrogenase,Citation128 (3) enolase catalyzing dehydration of 2-phosphoglycerate to phosphor-enolpyruvate,Citation96,Citation129,Citation130 and (4) Impdh, inosine 5-monophosphate dehydrogenase.Citation131 Five of these enzymes are directly or indirectly related to synthesis and/or modification of bacterial surface structure, including DltA, an enzyme catalyzing lipoteichoic d-alanylation,Citation132 PgdA, peptidoglycan N-acetylglucosamine deacetylase,Citation133 ApuA, a bifunctional amylopullulanase,Citation134 DPP IV, di-peptidyl peptidase IV,Citation135 and sortase A, a transpeptidase.Citation136,Citation137 One of the remaining two enzymes is IgA1 protease, which has highly immune-reactive activity to convalescent sera,Citation138 and the other is S-ribosylhomocycteinase (LuxS) catalyzing synthesis of auto-inducer 2 (AI-2) utilized in interspecies quorum sensing.Citation139,Citation140 As follows, we will discuss the roles of these enzymes in S. suis pathogenesis.
With the exceptions of neuB and neuC, the remaining six among the eight enzymes proposed by Wilson (including endo-β-N-acetylglucosaminidase [endo D], sucrose phosphorylase [gtfA], adenylosuccinate synthetase [purA], phosphoribosylamine-glycine ligase [purD], sucrose-6phosphate hydrolase [scrB], cytidine deaminase [cdd]) are poorly addressed and require further experimental validation.Citation100 NeuB is a sialic acid synthase that catalyzes the last committed step of the de novo biosynthetic pathway of sialic acid, a major element of bacterial surface structure. Recently, we systematically addressed its molecular and immunological role in bacterial virulence and claimed that an altered architecture of S. suis surface attenuates its virulence.Citation100,Citation141 Similarly, neuC that encodes UDP N-Acetylglucosamine 2-Epimerase with an involvement in sialic acid biosynthesis is also found to be essential for capsule production and required for virulence in a mouse infection model.Citation142
Gdh, the glutamine dehydrogenase-encoding gene, was originally known as a virulence factor. It has been widely applied to develop effective methods for differentiate and detect virulent Streptococcus suis species.Citation128 Si et al.Citation127 demonstrated that glutamine synthetase, the glnA-encoding product, is associated with S. suis virulence using a mouse model. We and two other research groupsCitation96,Citation129,Citation143 have reported that S. suis enolase acts as an octamer,Citation144 and can exported to the bacterial surface with capability of binding to host fibronectin, indicating its possible role in crosstalk between pathogen and host. However, the protective efficiency of recombinant enolase with different bacterial origins does not seem consistent.Citation96,Citation129,Citation130 Similarly, the gene encoding Inosine 5-monophosphate dehydrogenase, a nucleotide metabolism-related enzyme, was initially cloned by Lu’s research group, and was subsequently suggested to be involved in full virulence of SS2-H, a Chinese strain in the infection model of piglets.Citation131
Modification of the bacterial surface is critical for successful invasion and entry of pathogens into host cells. Gottschalk’s group systemically evaluated contributions of two kinds of modification systems to S. suis pathogenicity: lipoteichoic acid (LTA)-d-alanylation, and peptidoglycan (PG) N-deacetylation, respectively.Citation132,Citation133 The ΔdltA mutant with defection in LTA d-alanylation was found to be attenuated in its virulence, which can be correlated with its diminished adherence/invasion of porcine brain microvascular endothelial cells, and decreased capacity to escape immune clearance or killing by porcine neutrophils.Citation132 Fittipaldi et al.Citation133 observed that the expression level of the pgdA gene can be induced upon interaction of SS2 with neutrophils in vitro and infected mice in vivo, implying that PG N-deacetylation is tightly involved in SS2 infections. This hypothesis was further validated by virulence attenuation of the ΔpgdA mutant of SS2. Not only does ApuA behave like a bacterial surface protein with a LPKTGE cell-wall-anchoring motif at C-terminus, but it also functions as a bi-functional amylopullulanase.Citation134 Further genetic study showed that the multifunctional α-glucan-degrading enzyme ApuA can promote adhesion to porcine epithelium and mucus, which might link bacterial carbohydrate utilization to its capability of colonization and invasiveness into hosts. We and the other research group demonstrated that Sortase A (SrtA, originally referred to as a transpeptidase in Staphylococcus aureus) is essential for full virulence of SS2.Citation136,Citation137 However, other sortase paralogs SrtBCD are not associated with bacterial virulence.Citation145 In addition, we reported the functional definition of di-peptidyl peptidase IV (DPP IV) in S. suis 2, and also confirmed that it does contribute greatly to bacterial virulence.Citation135
Quorum sensing is a method of bacterial cell density-dependent communication, using secreted chemical molecules (like the auto-inducer) as a form of “language”. The luxS gene product is the synthetase of autoinducer 2 (AI-2) that is required for interspecies communication. Recently, we and Lu’s group both defined a functional LuxS member present in Chinese isolates of S. suis 2, and demonstrated its relevance to bacterial virulence.Citation139,Citation140 Zhang et al. identified that IgA1 protease, an immune-dominant antigen, is necessary for full virulence of S. suis 2.Citation138\ Additionally, Gruening et al.Citation146 proved that the arginine deiminase system (ADS), encoded by arcABC operon, is necessary for S. suis survival in acidic stress, indicating its possibility of correlating with bacterial successful infection.
Very recently, it was discovered that the adenosine synthase functions as an effector in evasion of PMNs-mediated innate immunityCitation147. It might suggest possibility that cyclic AMP (cAMP)-dependent signaling is linked to streptococcal infectivity. Given the fact that CadD enzyme, cAMP deaminase from Leptospira,Citation148 can quench cAMP-dependent signaling, it would be of interest to test the hypothesis that in vivo expression of CadD protein in S. suis modulate bacterial pathogenesis or not?
Transcriptional factors/regulators
No less than 16 pleiotropic regulators have been suggested to be involved in modulation of S. suis virulence, which consist of nine transcription factors (five well-studied ones [adcR,Citation149 ccpA,Citation118,Citation119 argR,Citation40 rgg,Citation31 a Fur-like repressor PerRCitation150] plus four poorly-known transcription factors such as 00SSU0053, treR, nadR, and scrRCitation100), five TCS systems (salK-salR [renamed as suiK-suiR],Citation27,Citation151 ciaR-ciaH,Citation42 ihk-ihr,Citation152 virR/virS,Citation153 and nisK-nisRCitation154), and three orphan regulators (CovR,Citation30 RevSC21,Citation155 and RevSCitation156,Citation157).
AdcR is a regulator controlling zinc transport in S. suis. Aranda and coauthors observed that disruption of this transcription factor can attenuate bacterial virulence in mouse model.Citation149 In contrast, Zur, the other zinc uptake regulator from 05ZYH33 strain of S. suis 2 is not essential for strong pathogenicity in porcine models.Citation29 Driven by the idea that host environment is critical for expression of bacterial virulence factors during the process of infection, Willenborg et al.Citation118 evaluated the effect of the sugar metabolism regulator catabolite control protein A (CcpA) on S. suis pathogenesis. As anticipated, expression levels of several virulence factors (such as ArcB, Sao, and Enolase) were altered in the ΔccpA mutant. Of particular note, a recent study implied that Sao protective antigen plays a limited role in bacterial virulence.Citation123 Moreover, the deletion of ccpA led to significant reduction of both capsule thickness and resistance to killing by porcine neutrophilsCitation118 and impaired bacterial virulence.Citation119 ArgR, a member of ArgR/AhrC arginine repressor family, was recently proved to regulate expression of arcABC operon encoding an arginine deiminase system that is recognized as a putative virulence factor.Citation40,Citation146 Therefore, it is of interest to test the role of argR in S. suis virulence. Similar to the scenario observed with Rgg regulators present in other gram-positive pathogen, Zheng et al. defined an rgg-like ortholog in S. suis 05ZYH33, and observed its multiple roles in bacterial metabolism. More importantly, it was verified to be a virulence determinant of S. suis 2 in experimental models of piglets.Citation31 Interestingly, an H2O2-responsive Fur-like regulator, PerR was confirmed to determine bacterial virulence through regulating expression of both dpr, a Dps-like peroxide resistance protein-encoding gene, and metQIN encoding a methionine transporter.Citation150
Among the 15 putative two-component signal transduction systems in Chinese virulent strain of S. suis 2,Citation23 five have been proposed to be correlated with manifestation of strong virulence.Citation27,Citation42,Citation152,Citation153 In 2008, we reported the salK-salR system present in the 89K pathogenicity island. The deletion of this TCS system resulted in significant downregulation of 26 genes’ expression level, and increased its susceptibility to polymorphonuclear leukocyte (PMN)-mediated killing. Consequently, the virulence of the ΔsalK-R mutant was seriously attenuated.Citation27 Shen et al.Citation158 conducted a follow-up study to address the regulatory network of SalK-SalR TCS system. As expected, proteomics-based investigation revealed 14 downregulated proteins and 1 upregulated protein, which partially agreed with our former microarray analysis.Citation158 To our much surprise, Zhong’s group very recently elucidated the physiological role of this SalK-SalR TCS in regulating a bioactive lantibiotic suicin production, thereby replaced it with SuiK-SuiR.Citation151
Li and coworkersCitation42 reported the second TCS system, ciaK-ciaR, which is required for pathogenicity of SS2 in the infection models of both CD1 mice and piglets. Han et al. showed that Ihk/Irr TCS contributes to full virulence of Streptococcus suis serotype 2 strain 05ZYH33 via regulating bacterial cell metabolism.Citation152 Very recently, two more TCS (VirR/VirSCitation153 and NisK/RCitation154) was demonstrated to be essential for SS2 pathogenicity.
Also, an orphan response regulator revSC21 was determined by the same research group.Citation155 This regulator revSC21 positively modulates expression levels of virulence factors (e.g., mrp, sly, and cps) and is required for bacterial pathogenesis.Citation155 In contrast, Pan et al. reported another orphan response regulator CovR with an opposite effect on S. suis pathogenicity.Citation30 The covR-defective (ΔcovR) mutant displayed thicker capsules and increased hemolytic activity. Furthermore, adherence of this mutant to epithelial cells was greatly increased, as well as its resistance to phagocytosis and killing by neutrophils and monocytes. Eventually, the removal of covR gene was found to be correlated with increased lethality of piglets, relative to those inoculated with its parent virulent strain 05ZYH33.Citation30
Others
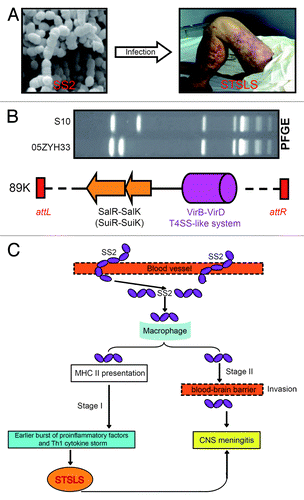
VirA is a newly-determined virulence factor that is exclusively present in virulent SS2 strain.Citation122 Trigger factor is also a virulence determinant which acts by controlling expression of a collection of known virulence factors, such as cps, mrp, and sly.Citation159 FeoBA encoding an iron transporter system might represent the first example of the fact that the machinery of a transporter can be involved in S. suis pathogenicity.Citation35 After Li et al.Citation28 defined transposal characteristics of 89K PAI in epidemic strain of Chinese virulent SS2, Hu’s group recently obtained further insights into 89K PAI. In addition to virulence-determining element, salK-salR TCS system, 89K PAI also carried a second virulence factor, virD4-virB4, a type IV-like secretion system. Unexpectedly, this T4SS-like system is responsible for stimulating host immune reaction observed in mice infected with Chinese streptococcal toxic shock-like syndrome (STSLS)-causing strain of S. suis 2 Citation160, raising a possible relationship of 89K to molecular/immunological machinery by which this non-group A streptococcus (GAS), SS2 to cause STSLS in infected patients (). Finally, Wilson et al. also suggested that 05SSU0660, an uncharacterized protein homologous to S. pneumonia spr1018 might be a virulence factor.Citation100
Figure 4. Clinical syndromes, genetic basis and working model for streptococcal toxic shock-like syndrome (STSLS) caused by S. suis 2. (A) Clinical visualization of a representative SS2-infected patient with streptococcal toxic shock-like syndrome. It clearly shows purpura and gangrenous changes on this patient’s legs. Partially adapted from reference Citation9 with permission. (B) Genetic evidence that 89K pathogenicity island carries elements associated with bacterial virulence and the clinical consequence of streptococcal toxic shock-like syndrome. PFGE assay reveals Chinese epidemic strain 05ZYH33 is distinct from the international strain S10.Citation4 The two genetic elements of the transposable 89K pathogenicity islandCitation23,Citation28 have been functionally defined: one is SalK-SalR (renamed as SuiK-SuiRCitation151) TCS with requirement for full virulence,Citation27 and other is VirD4-VirB4 T4SS-like system associated with clinical manifestation of STSLS in mouse model.Citation160 (C) The proposed model of the two-stage hypothesis for STSLS. SS2 gets into blood vessels in stage I and results in an early burst of proinflammatory factors and Th1 cytokine storms. Such kind of inflammatory super-responses lead to STSLS with death as early as 13 h after SS2 infection. In stage II (i.e., several days post-infection), SS2 might use virulence factors like suilysin to cause disease, particularly meningitis.Citation94 CNS, central nervous system. Integrated and modified from references Citation4, Citation23, Citation27, Citation28, Citation94, and Citation160 with permission.
Host Immunological/Inflammatory Factors
Pathogens have evolved capacity to recruit/hijack host cell factors for their successful infections/invasions. So far, the majority of host cell factors identified to be involved in S. suis pathogenesis are immunological/apoptotic/inflammatory factors.
Nearly ten years ago, Gottschalk et al.Citation161 had observed that cell wall components of S. suis can induce releases of interleukin-1 (IL-1), IL-8, monocyte chemotactic protein-1 (MCP-1) in human brain microvascular endothelial cells (BMEC), which might increase the permeability of the blood-brain barrier. Using the experimental model of CD1 mice, the same group observed that (1) ~20% animals with sudden death exhibit high levels of systemic TNF-α, IL-6, IL-12, IFN-γ, CCL2, CXCL1, and CCL5 24 h after infection; (2) infected mice that survived the early sepsis later developed clinical signs of meningitis present in the transcriptional activation of TLR2, TLR3, CD14, NFκB, IL-1β, CCL2, and TNF-α, mainly in myeloid cells located in affected cerebral structures.Citation162 Apparently, the inflammatory response plays important roles in S. suis infection of CD1 mice. Similar observation was also reported by Scherk et al.Citation163 that S. suis infections are correlated with the release of pro-inflammatory cytokines and chemokines (e.g., IL6 and IL8). Gottschalk et al. found that the toll-like receptor 2 (TLR-2)-deficient exhibit significantly reduced production of astrocytes,Citation164 and proposed that bacterial polysaccharides probably modulates TLR2-dependent recognition of S. suis entry/invasiveness into CD1 mice.Citation165
Using a transcriptomic approach, de Greeff et al.Citation166 identified macrophage-specific genes (IL-1-β, MIP-2-α, and TNF-α) with significantly different expression upon S. suis infection, suggesting that MAP-kinase signaling pathway and NFκB signaling are implicated into response of porcine alveolar macrophages to S. suis infections. Additionally, Wu et al.Citation167 verified that a novel murine ribonuclease, angiogenin inhibitor 1 (AI1) can bind to S. suis hyaluronidase (Hyl), and hypothesized that this interaction between host AI1 partner and bacterial Hyl protein might contribute to S. suis meningitis.
Protective Antigens
Development of a safe and efficient vaccine is a useful strategy to combat against S. suis infection. In contrast to conventional killed/live whole-bacteria vaccines,Citation168-Citation170 engineering of subunit vaccine exhibits significant advantage in its safety and its large-scale producibility.Citation171 Identification of protective antigens is a prerequisite for identifying candidate vaccine molecules. Totally, there are no less than 15 protective antigens identified thus far (). Among them, four molecules of protective antigen are well-known virulence associated factors and are MRP, EF, 38 kDa, and SLY (). Of note, most of the remaining 10 protein antigens were elucidated by research groups in China. In 2009, in addition to identification of the known protective antigen SLY, Liu’s report verified three more new protective antigens that are RTX family exoprotein A (RfeA), epidermal surface antigen (ESA), and immunoglobulin G (IgG)-binding protein (IBP).Citation172 Two different research groups from China confirmed that enolase, an enzyme of central metabolism, acts as a protective antigen displayed on bacterial surface.Citation96,Citation129 However, Esgleas and coworkerCitation130 reported an opposite result regarding the protective efficiency of enolase in mice. This discrepancy could be due to different versions of recombinant protein, different strains plus deviations in animal vaccination protocols. In particular note, all the three newly-identified immunogenic antigens (6PGD, HP0197, and HP245) are elucidated by the same Chen’s research group in China (). Interestingly, proteomics also facilitated to discover three new immunogenic proteins SsPepO,Citation173 Sat (HP0272),Citation112,Citation174 and the pilus subunit PAPI-2bCitation125 (). In disagreement with their former observation,Citation121 Li and coworkersCitation122 demonstrated that SAO surface antigen can confer efficient protection in both mice and piglets against virulent SS2 infection ().
Table 3. List of protective antigens and/or candidate vaccine molecules from Streptococcus suis
Zoonotic Potential of S. suis
Clinical consequence of human SS2 infections
It is accepted that S. suis has developed into a significant human pathogen, especially in southeast Asia, posing a great challenge to public health.Citation2 Clinically, a collection of disease types can be observed in those patients with S. suis infections, including meningitis, septicemia, and pneumonia. In terms of epidemiological/clinical statistics, bacterial meningitis is the most prevalent symptom caused by S. suis infection.
Meningitis
Meningitis is medically defined as an inflammation of the lining that covers the brain and spinal cord (the meninges).Citation10,Citation16,Citation60 This kind of inflammation usually initiates with a brief influenza-like prodroma and results in hearing impairment or loss.Citation77,Citation175 Generally, meningitis can be grouped into two types, bacterial and non-bacterial (e.g., viral or fungal meningitis).Citation77 For the former, no less than 10 kinds of bacterial pathogens (such as Mycobacteria tuberculosis, Streptococcus pneumonia, and Staphylococcus aureus) have been determined as the causatives of development of human meningitis.Citation33,Citation77
Although SS2 is a swine pathogen, we have come to know that SS2 can also be a severe agent for human bacterial meningitis.Citation60,Citation88 The first case of human SS2 infection worldwide was recorded in Denmark in 1968.Citation1 Hui et al. declared that SS2 would be another leading cause of the so-called community-acquired meningitis, which is only inferior to M. tuberculosis and S. pneumonia.Citation77 A systematic survey of bacterial meningitis in Vietnamese adults recently suggested that (1) SS2 with multiple virulence factors (e.g., EPF, SLY, MRP) is the most common pathogen and (2) its mortality is relatively low (2.6%), but hearing loss occurs at high percentage (66.4%).Citation16 Moreover, Hoa et al.Citation176 addressed the antimicrobial susceptibility profile of S. suis strains from meningitis patients, an important question, using comprehensive approaches to analyze bacterial isolates in Vietnam from 1997 to 2008. As result, they found that (1) multidrug resistance in S. suis 2 causing meningitis in southern Vietnam has increased over the 11-y period studied; (2) the tet(L) carried in these bacteria is functionally expressed, and multiple other genes are probably co-expressed.Citation176 These findings alerted us again that it is important to minimize abuse of antibiotics in treatment of bacterial infectious diseases, and that the development of new therapeutics against SS2 infections are in great demand. Fortunately, Ho et al.Citation177 reported the largest prospective epidemiological survey of human SS2 meningitis in Vietnam, and pointed out that three important risk factors associated with human SS2 meningitis included (1) eating “high risk” dishes popular in southeast Asia, (2) occupational exposure to pigs and/or pork-related products, and (3) preparation of pork in the presence of skin lesions. Further investigations from the same research group demonstrated that slaughterhouse pigs are a major reservoir of SS2 that led to human infections of SS2 meningitis in southern Vietnam.Citation178 It highlighted (1) the importance of an improved hygiene at pork processing facilities, and (2) the necessity for education programs concerning food safety and proper handling of pork.Citation178
Similar findings were also noted in the NetherlandsCitation59 and New Zealand.Citation86,Citation179 In the Netherlands, researchers have clearly indicated that the risk of developing SS2 meningitis among abattoir workers, butchers, and pig breeders are above 1000 times higher than that among persons without close contact with pigs or their unprocessed pork products.Citation59 To our surprise, a study performed in New Zealand revealed that a high ratio of farmers and meat inspectors in markets were sero-positive to SS2, suggesting the presence of human sub-clinical SS2 infections to a small extent.Citation86,Citation179
Septicemia
Septicemia is a serious and life-threatening infection, in which a large amount of bacteria are present in the blood. It is commonly referred to as “blood poisoning” or “bacteremia with sepsis”.Citation1 This disease often causes multi-organ failure by reducing the amount of blood reaching vital organs such as the liver and kidneys.Citation1,Citation48,Citation75 In addition to the known bacterial pathogens (such as Staphylococcus) with capability of leading to septicemia with about 25% lethal rate,Citation1,Citation180 SS2 is also a causative agent of this disease.Citation1 In general, SS2-caused septicemia arises as a result of the localized infection in the body, especially cut skin.Citation1 In patients with septicemia, some of the following symptoms can be observed: fever and chills, rising heart/respiratory rate, cold and clammy feeling, fallen blood pressure, paled ,and petechial skin, and ultimately even unconsciousness. SS2 most likely releases some toxins into the blood that break down the walls of blood vessels which allows blood to leak out under the skin (it is this leaking that causes the rash or petechiae).Citation33 In some cases, SS2 infects the bloodstream and the meninges at the same time, causing both septicemia and meningitis.Citation33,Citation181 Due to the rapidly progressive condition of septicemia, it can evolve into an irreversible toxic shock and even acute death if sufferers do not receive urgent treatment.Citation33,Citation181,Citation182 Certainly, an antibiotic remedy could be altered quickly to treat the target bacteria agents, when medical tests have identified which bacteria cause the septicemia and which antibiotics are best effective.Citation33,Citation182 Of note, some medical treatments themselves are the inducers of septicemia, e.g., dental treatment, long-term use of intravenous needles, a colostomy, and so on.Citation33,Citation175 Collectively, septicemia is so serious and complex that it deserves a lot of attention worldwide.
Others
In addition to the above two major forms of SS2-caused diseases, there are some other clinical types that occur less frequently in the cases of human SS2 infections, including arthritis, endocarditis, streptococcal toxic shock-like syndrome (STSLS), etc. Since STSLS is a newly-described disease form of SS2 infections (), here we have discussed it in detail. Toxic-shock syndrome refers to a highly invasive infection of deep tissues, and can be correlated with production of bacterial super-antigens (e.g., staphylococcal and streptococcal exotoxins).Citation183,Citation184 Before Tang et al.Citation18 formally proposed that SS2 is another bacterial non-GAS agent responsible for STSLS, we had been aware that StaphylococcusCitation185,Citation186 and Streptococcus pyogenes, a group A streptococcus (GAS)Citation183,Citation184 are both leading pathogens with a capability of causing STSLS. The clinical criteria for diagnosis of STSLS disease can be described as follows: (1) clear erythematous blanching rash (), (2) sudden onset of high fever, (3) hypotension diarrhea, (4) blood spots and petechiae, and (5) dysfunction of multiple organs (e.g., disseminated intravascular coagulation and acute renal failure).Citation8,Citation9,Citation18 According to above clinical criteria, two independent research groups in China reported two big outbreaks of human SS2-caused STSLS, which occurred in Jiangsu Province, 1998, and Sichuan Province, 2005, respectively.Citation9,Citation17-Citation19,Citation34 Two models have been proposed to explain the molecular mechanism by which SS2 triggers STSLS in its infected patients, one of which is a two-stage hypothesis,Citation94 the other is a specific 89K PAI.Citation23,Citation160 Although we came to know that similar findings were also recorded in Thailand,Citation187 France,Citation188 and Australia,Citation84 we did not gain any information on difference of these SS2 strains abroad in comparison with Chinese STSLS-causing isolates. Given that the above two models are based on evidence from studying the Chinese SS2 strains, we can’t conclude presently that they represent common or strain-specific mechanisms.
Two kinds of non-SS2 serotypes have been determined in sporadic cases of human S. suis infections: One of these is SS14,Citation13 and the other is SS16.Citation16 Poggenborg and coworkerCitation189 reported a pig-farm worker with SS14 meningitis and septicaemia complicated with thoracal and lumbar spine spondylodiscitis. Similarly, Ahmed et al.Citation13 also documented a meningitis case caused by SS14 infections, in a female patient with occupational exposure to piglets each day. Of particular note, totally 12 cases of human SS14 infections were identified in Thailand during 2006–2008, and their clinical presentations included meningitis, septic arthritis and sepsis.Citation14 Further analyses demonstrated that 11 of the 12 SS14 isolates from these patients belonged to the multilocus sequence types (ST) 105, suggesting clonal dissemination of ST105 strains in Thailand. In another neighboring Asian country, Vietnam, a fatal case of human SS16 infection was revealed by Schultsz’s research group,Citation16 indicating multiple serotypes of S. suis are developing into human pathogens circulating in this country. Together, non-SS2 serotypes of S. suis have also exhibited its zoonotic potential, highlighting a great demand for monitoring their epidemiology and development of relevant approaches applied toward prevention and therapeutics.
Concluding Remarks and Perspectives
As a zoonotic agent, S. suis is gathering increasing accumulated attention from public health officials as well as the relevant academic community.Citation2,Citation3,Citation8 With respect to S. suis epidemiology, we believe that current situation of human SS2 infections should be re-evaluated using comprehensive approaches. To minimize the occupational infections by S. suis, it is suggested that advances be made to improve public awareness of S. suis infection by science education and popularization. Although progress has been obtained toward understanding S. suis pathogenicity (especially identifying a group of bacterial virulence factors), it still sounds fragmentary, and lacks an insightful dissection of integrated regulatory networks of bacterial virulence. In the future, an important direction would be to link posttranscriptional regulation (e.g., ncRNA and riboswitch) and modification (such as acetylation and de-acetylation) to the bacterial virulence of Streptococcus suis.Citation190 Moreover, structural information on these virulence factors is very limited (). We therefore believe it is necessary to strengthen the study of pathogenesis-related structural biology, which could establish a solid basis for design of small molecule drugs targeting bacterial virulence factors.
It is of interest to search for other virulence-related or immunological regulatory elements, different from 89K PAI, because of recent exciting findings on this issue.Citation160 Safe and effective vaccines that can be used for patients is still not available, which is due to lack of comprehensive knowledge of S. suis infections. Description of a full picture of S. suis surface antigen proteins might be helpful to screen and/or design vaccine molecules candidate. The fact that non-SS2 serotypes of S. suis can affect humans and even lead to fatal infections, has put the situation of S. suis infection in a much more complicated and serious status in public health. This might be an alternate way to decode the genome sequences of all 35 kinds of different serotypes, providing new insights into evolution and diversity of heterogeneous species, as well as distinct clues for preventing and controlling severe infections by these pathogens.Citation191,Citation192 Systematic proteomic approaches are also encouraged to further address this question, which can complement genomics-based explorations.Citation138
In summary, our understanding and response to the situation of S. suis infections occurring especially in southeast Asia is not satisfactory. It would be helpful to integrate representative virulent/avirulent strains from different countries/regions for collaborative investigations. Therefore, there is a long way to go toward the complete conquest of S. suis, an emerging human pathogen.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize for not citing all relevant important references due to the space limit. We would like to thank Mr Derek Caetano-Anolles and Dr Swaminath Srinivas for the critical reading of this manuscript. It was partially supported by the startup package of Zhejiang University, China, the National Natural Science Foundation of China (31170124, 81371768, 81172794 and 31300119), National S&T Project for Infectious Diseases Control (2013ZX10004-103, 2013ZX10004-203, and 2013ZX10004-218). Y.F. is a recipient of the “Young 1000 Talents” Award, and has also been awarded as a visiting scholar in Department of Epidemiology, Research Institute for Medicine of Nanjing Command.
References
- Staats JJ, Feder I, Okwumabua O, Chengappa MM. Streptococcus suis: past and present. Vet Res Commun 1997; 21:381 - 407; http://dx.doi.org/10.1023/A:1005870317757; PMID: 9266659
- Wertheim HF, Nghia HD, Taylor W, Schultsz C. Streptococcus suis: an emerging human pathogen. Clin Infect Dis 2009; 48:617 - 25; http://dx.doi.org/10.1086/596763; PMID: 19191650
- Gottschalk M, Segura M, Xu J. Streptococcus suis infections in humans: the Chinese experience and the situation in North America. Anim Health Res Rev 2007; 8:29 - 45; http://dx.doi.org/10.1017/S1466252307001247; PMID: 17692141
- Feng Y, Shi X, Zhang H, Zhang S, Ma Y, Zheng B, Han H, Lan Q, Tang J, Cheng J, et al. Recurrence of human Streptococcus suis infections in 2007: three cases of meningitis and implications that heterogeneous S. suis 2 circulates in China. Zoonoses Public Health 2009; 56:506 - 14; http://dx.doi.org/10.1111/j.1863-2378.2008.01225.x; PMID: 19538458
- Gottschalk M, Xu J, Calzas C, Segura M. Streptococcus suis: a new emerging or an old neglected zoonotic pathogen?. Future Microbiol 2010; 5:371 - 91; http://dx.doi.org/10.2217/fmb.10.2; PMID: 20210549
- Suankratay C, Intalapaporn P, Nunthapisud P, Arunyingmongkol K, Wilde H. Streptococcus suis meningitis in Thailand. Southeast Asian J Trop Med Public Health 2004; 35:868 - 76; PMID: 15916083
- Wertheim HF, Nguyen HN, Taylor W, Lien TT, Ngo HT, Nguyen TQ, Nguyen BN, Nguyen HH, Nguyen HM, Nguyen CT, et al. Streptococcus suis, an important cause of adult bacterial meningitis in northern Vietnam. PLoS One 2009; 4:e5973; http://dx.doi.org/10.1371/journal.pone.0005973; PMID: 19543404
- Feng Y, Zhang H, Ma Y, Gao GF. Uncovering newly emerging variants of Streptococcus suis, an important zoonotic agent. Trends Microbiol 2010; 18:124 - 31; http://dx.doi.org/10.1016/j.tim.2009.12.003; PMID: 20071175
- Yu H, Jing H, Chen Z, Zheng H, Zhu X, Wang H, Wang S, Liu L, Zu R, Luo L, et al, Streptococcus suis study groups. Human Streptococcus suis outbreak, Sichuan, China. Emerg Infect Dis 2006; 12:914 - 20; http://dx.doi.org/10.3201/eid1206.051194; PMID: 16707046
- Arends JP, Zanen HC. Meningitis caused by Streptococcus suis in humans. Rev Infect Dis 1988; 10:131 - 7; http://dx.doi.org/10.1093/clinids/10.1.131; PMID: 3353625
- Kerdsin A, Dejsirilert S, Sawanpanyalert P, Boonnark A, Noithachang W, Sriyakum D, Simkum S, Chokngam S, Gottschalk M, Akeda Y, et al. Sepsis and spontaneous bacterial peritonitis in Thailand. Lancet 2011; 378:960; http://dx.doi.org/10.1016/S0140-6736(11)60923-9; PMID: 21890062
- Gustavsson C, Ramussen M. Septic arthritis caused by Streptococcus suis serotype 5 in pig farmer. Emerg Infect Dis 2014; 20:489 - 91; http://dx.doi.org/10.3201/eid2003.130535; PMID: 24565084
- Haleis A, Alfa M, Gottschalk M, Bernard K, Ronald A, Manickam K. Meningitis caused by Streptococcus suis serotype 14, North America. Emerg Infect Dis 2009; 15:350 - 2; http://dx.doi.org/10.3201/eid1502.080842; PMID: 19193296
- Kerdsin A, Oishi K, Sripakdee S, Boonkerd N, Polwichai P, Nakamura S, Uchida R, Sawanpanyalert P, Dejsirilert S. Clonal dissemination of human isolates of Streptococcus suis serotype 14 in Thailand. J Med Microbiol 2009; 58:1508 - 13; http://dx.doi.org/10.1099/jmm.0.013656-0; PMID: 19661209
- Nghia HD, Hoa NT, Linh D, Campbell J, Diep TS, Chau NV, Mai NT, Hien TT, Spratt B, Farrar J, et al. Human case of Streptococcus suis serotype 16 infection. Emerg Infect Dis 2008; 14:155 - 7; http://dx.doi.org/10.3201/eid1401.070534; PMID: 18258097
- Mai NT, Hoa NT, Nga TV, Linh D, Chau TT, Sinh DX, Phu NH, Chuong LV, Diep TS, Campbell J, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis 2008; 46:659 - 67; http://dx.doi.org/10.1086/527385; PMID: 19413493
- Ye C, Zhu X, Jing H, Du H, Segura M, Zheng H, Kan B, Wang L, Bai X, Zhou Y, et al. Streptococcus suis sequence type 7 outbreak, Sichuan, China. Emerg Infect Dis 2006; 12:1203 - 8; http://dx.doi.org/10.3201/eid1708.060232; PMID: 16965698
- Tang J, Wang C, Feng Y, Yang W, Song H, Chen Z, Yu H, Pan X, Zhou X, Wang H, et al. Streptococcal toxic shock syndrome caused by Streptococcus suis serotype 2. PLoS Med 2006; 3:e151; http://dx.doi.org/10.1371/journal.pmed.0030151; PMID: 16584289
- Ma Y, Feng Y, Liu D, Gao GF. Avian influenza virus, Streptococcus suis serotype 2, severe acute respiratory syndrome-coronavirus and beyond: molecular epidemiology, ecology and the situation in China. Philos Trans R Soc Lond B Biol Sci 2009; 364:2725 - 37; http://dx.doi.org/10.1098/rstb.2009.0093; PMID: 19687041
- Smith HE, Damman M, van der Velde J, Wagenaar F, Wisselink HJ, Stockhofe-Zurwieden N, Smits MA. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun 1999; 67:1750 - 6; PMID: 10085014
- Smith HE, Vecht U, Wisselink HJ, Stockhofe-Zurwieden N, Biermann Y, Smits MA. Mutants of Streptococcus suis types 1 and 2 impaired in expression of muramidase-released protein and extracellular protein induce disease in newborn germfree pigs. Infect Immun 1996; 64:4409 - 12; PMID: 8926123
- Jacobs AA, van den Berg AJ, Loeffen PL. Protection of experimentally infected pigs by suilysin, the thiol-activated haemolysin of Streptococcus suis.. Vet Rec 1996; 139:225 - 8; http://dx.doi.org/10.1136/vr.139.10.225; PMID: 8883345
- Chen C, Tang J, Dong W, Wang C, Feng Y, Wang J, Zheng F, Pan X, Liu D, Li M, et al. A glimpse of streptococcal toxic shock syndrome from comparative genomics of S. suis 2 Chinese isolates. PLoS One 2007; 2:e315; http://dx.doi.org/10.1371/journal.pone.0000315; PMID: 17375201
- Holden MT, Hauser H, Sanders M, Ngo TH, Cherevach I, Cronin A, Goodhead I, Mungall K, Quail MA, Price C, et al. Rapid evolution of virulence and drug resistance in the emerging zoonotic pathogen Streptococcus suis.. PLoS One 2009; 4:e6072; http://dx.doi.org/10.1371/journal.pone.0006072; PMID: 19603075
- Hu P, Yang M, Zhang A, Wu J, Chen B, Hua Y, Yu J, Chen H, Xiao J, Jin M. Complete genome sequence of Streptococcus suis serotype 3 strain ST3. J Bacteriol 2011; 193:3428 - 9; http://dx.doi.org/10.1128/JB.05018-11; PMID: 21572001
- Hu P, Yang M, Zhang A, Wu J, Chen B, Hua Y, Yu J, Xiao J, Jin M. Complete genome sequence of Streptococcus suis serotype 14 strain JS14. J Bacteriol 2011; 193:2375 - 6; http://dx.doi.org/10.1128/JB.00083-11; PMID: 21398551
- Li M, Wang C, Feng Y, Pan X, Cheng G, Wang J, Ge J, Zheng F, Cao M, Dong Y, et al. SalK/SalR, a two-component signal transduction system, is essential for full virulence of highly invasive Streptococcus suis serotype 2. PLoS One 2008; 3:e2080; http://dx.doi.org/10.1371/journal.pone.0002080; PMID: 18461172
- Li M, Shen X, Yan J, Han H, Zheng B, Liu D, Cheng H, Zhao Y, Rao X, Wang C, et al. GI-type T4SS-mediated horizontal transfer of the 89K pathogenicity island in epidemic Streptococcus suis serotype 2. Mol Microbiol 2011; 79:1670 - 83; http://dx.doi.org/10.1111/j.1365-2958.2011.07553.x; PMID: 21244532
- Feng Y, Li M, Zhang H, Zheng B, Han H, Wang C, Yan J, Tang J, Gao GF. Functional definition and global regulation of Zur, a zinc uptake regulator in a Streptococcus suis serotype 2 strain causing streptococcal toxic shock syndrome. J Bacteriol 2008; 190:7567 - 78; http://dx.doi.org/10.1128/JB.01532-07; PMID: 18723622
- Pan X, Ge J, Li M, Wu B, Wang C, Wang J, Feng Y, Yin Z, Zheng F, Cheng G, et al. The orphan response regulator CovR: a globally negative modulator of virulence in Streptococcus suis serotype 2. J Bacteriol 2009; 191:2601 - 12; http://dx.doi.org/10.1128/JB.01309-08; PMID: 19181815
- Zheng F, Ji H, Cao M, Wang C, Feng Y, Li M, Pan X, Wang J, Qin Y, Hu F, et al. Contribution of the Rgg transcription regulator to metabolism and virulence of Streptococcus suis serotype 2. Infect Immun 2011; 79:1319 - 28; http://dx.doi.org/10.1128/IAI.00193-10; PMID: 21149588
- Gottschalk M, Segura M. The pathogenesis of the meningitis caused by Streptococcus suis: the unresolved questions. Vet Microbiol 2000; 76:259 - 72; http://dx.doi.org/10.1016/S0378-1135(00)00250-9; PMID: 10973700
- Huang YT, Teng LJ, Ho SW, Hsueh PR. Streptococcus suis infection. J Microbiol Immunol Infect 2005; 38:306 - 13; PMID: 16211137
- Ye C, Bai X, Zhang J, Jing H, Zheng H, Du H, Cui Z, Zhang S, Jin D, Xu Y, et al. Spread of Streptococcus suis sequence type 7, China. Emerg Infect Dis 2008; 14:787 - 91; http://dx.doi.org/10.3201/eid1405.070437; PMID: 18439362
- Aranda J, Cortés P, Garrido ME, Fittipaldi N, Llagostera M, Gottschalk M, Barbé J. Contribution of the FeoB transporter to Streptococcus suis virulence. Int Microbiol 2009; 12:137 - 43; PMID: 19784934
- Feng Y, Zheng F, Pan X, Sun W, Wang C, Dong Y, Ju AP, Ge J, Liu D, Liu C, et al. Existence and characterization of allelic variants of Sao, a newly identified surface protein from Streptococcus suis.. FEMS Microbiol Lett 2007; 275:80 - 8; http://dx.doi.org/10.1111/j.1574-6968.2007.00859.x; PMID: 17854470
- Ngo TH, Tran TB, Tran TT, Nguyen VD, Campbell J, Pham HA, Huynh HT, Nguyen VV, Bryant JE, Tran TH, et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One 2011; 6:e17943; http://dx.doi.org/10.1371/journal.pone.0017943; PMID: 21464930
- Nghia HD, Tu TP, Wolbers M, Thai CQ, Hoang NV, Nga TV, Thao TP, Phu NH, Chau TT, Sinh DX, et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One 2011; 6:e17604; http://dx.doi.org/10.1371/journal.pone.0017604; PMID: 21408132
- Feng Y, Cao M, Wu Z, Chu F, Ma Y, Wang C, Zhang H, Pan X, Mao X, Zou Q.. Streptococcus suis in Omics-Era: Where do we stand?. J Bacteriol Parasitol 2011; S2:001
- Fulde M, Willenborg J, de Greeff A, Benga L, Smith HE, Valentin-Weigand P, Goethe R. ArgR is an essential local transcriptional regulator of the arcABC operon in Streptococcus suis and is crucial for biological fitness in an acidic environment. Microbiology 2011; 157:572 - 82; http://dx.doi.org/10.1099/mic.0.043067-0; PMID: 20947575
- Bonifait L, Grenier D. The SspA subtilisin-like protease of Streptococcus suis triggers a pro-inflammatory response in macrophages through a non-proteolytic mechanism. BMC Microbiol 2011; 11:47; http://dx.doi.org/10.1186/1471-2180-11-47; PMID: 21362190
- Li J, Tan C, Zhou Y, Fu S, Hu L, Hu J, Chen H, Bei W. The two-component regulatory system CiaRH contributes to the virulence of Streptococcus suis 2. Vet Microbiol 2011; 148:99 - 104; http://dx.doi.org/10.1016/j.vetmic.2010.08.005; PMID: 20832201
- Jing HB, Yuan J, Wang J, Yuan Y, Zhu L, Liu XK, Zheng YL, Wei KH, Zhang XM, Geng HR, et al. Proteome analysis of Streptococcus suis serotype 2. Proteomics 2008; 8:333 - 49; http://dx.doi.org/10.1002/pmic.200600930; PMID: 18081191
- Zhang W, Lu CP. Immunoproteomic assay of membrane-associated proteins of Streptococcus suis type 2 China vaccine strain HA9801. Zoonoses Public Health 2007; 54:253 - 9; http://dx.doi.org/10.1111/j.1863-2378.2007.01056.x; PMID: 17803514
- Zhang W, Lu CP. Immunoproteomics of extracellular proteins of Chinese virulent strains of Streptococcus suis type 2. Proteomics 2007; 7:4468 - 76; http://dx.doi.org/10.1002/pmic.200700294; PMID: 18022935
- Wu Z, Zhang W, Lu C. Comparative proteome analysis of secreted proteins of Streptococcus suis serotype 9 isolates from diseased and healthy pigs. Microb Pathog 2008; 45:159 - 66; http://dx.doi.org/10.1016/j.micpath.2008.04.009; PMID: 18554861
- Wu Z, Zhang W, Lu C. Immunoproteomic assay of surface proteins of Streptococcus suis serotype 9. FEMS Immunol Med Microbiol 2008; 53:52 - 9; http://dx.doi.org/10.1111/j.1574-695X.2008.00401.x; PMID: 18371070
- Lun ZR, Wang QP, Chen XG, Li AX, Zhu XQ. Streptococcus suis: an emerging zoonotic pathogen. Lancet Infect Dis 2007; 7:201 - 9; http://dx.doi.org/10.1016/S1473-3099(07)70001-4; PMID: 17317601
- Willenburg KS, Sentochnik DE, Zadoks RN. Human Streptococcus suis meningitis in the United States. N Engl J Med 2006; 354:1325; http://dx.doi.org/10.1056/NEJMc053089; PMID: 16554543
- Lee GT, Chiu CY, Haller BL, Denn PM, Hall CS, Gerberding JL. Streptococcus suis meningitis, United States. Emerg Infect Dis 2008; 14:183 - 5; http://dx.doi.org/10.3201/eid1401.070930; PMID: 18258107
- Trottier S, Higgins R, Brochu G, Gottschalk M. A case of human endocarditis due to Streptococcus suis in North America. Rev Infect Dis 1991; 13:1251 - 2; http://dx.doi.org/10.1093/clinids/13.6.1251; PMID: 1775866
- Michaud S, Duperval R, Higgins R. Streptococcus suis meningitis: First case reported in Quebec. Can J Infect Dis 1996; 7:329 - 31; PMID: 22514459
- Lopreto C, Lopardo HA, Bardi MC, Gottschalk M. [Primary Streptococcus suis meningitis: first case in humans described in Latin America.]. Enferm Infecc Microbiol Clin 2005; 23:110; http://dx.doi.org/10.1157/13071618; PMID: 15743586
- Callejo R, Prieto M, Salamone F, Auger JP, Goyette-Desjardins G, Gottschalk M. Atypical Streptococcus suis in man, Argentina, 2013. Emerg Infect Dis 2014; 20:500 - 2; http://dx.doi.org/10.3201/eid2003.131148; PMID: 24565286
- Koch E, Fuentes G, Carvajal R, Palma R, Aguirre V, Cruz C, Henríquez R, Calvo M. [Streptococcus suis meningitis in pig farmers: report of first two cases in Chile]. Rev Chilena Infectol 2013; 30:557 - 61; http://dx.doi.org/10.4067/S0716-10182013000500015; PMID: 24248173
- Demar M, Belzunce C, Simonnet C, Renaux A, Abboud P, Okandze A, Marois-Créhan C, Djossou F. Streptococcus suis meningitis and bacteremia in man, French Guiana. Emerg Infect Dis 2013; 19:1545 - 6; http://dx.doi.org/10.3201/eid1909.121872; PMID: 23977863
- Geffner Sclarsky DE, Moreno Muñoz R, Campillo Alpera MS, Pardo Serrano FJ, Gómez Gómez A, Martínez-Lozano MD. [Streptococcus suis meningitis]. An Med Interna 2001; 18:317 - 8; http://dx.doi.org/10.4321/S0212-71992001000600007; PMID: 11503579
- van de Beek D, Spanjaard L, de Gans J. Streptococcus suis meningitis in the Netherlands. J Infect 2008; 57:158 - 61; http://dx.doi.org/10.1016/j.jinf.2008.04.009; PMID: 18538852
- Halaby T, Hoitsma E, Hupperts R, Spanjaard L, Luirink M, Jacobs J. Streptococcus suis meningitis, a poacher’s risk. Eur J Clin Microbiol Infect Dis 2000; 19:943 - 5; http://dx.doi.org/10.1007/PL00011230; PMID: 11205632
- de Ceuster LM, van Dillen JJ, Wever PC, Rozemeijer W, Louwerse ES. [Streptococcus suis meningitis in a meat factory employee]. Ned Tijdschr Geneeskd 2012; 156:A5080; PMID: 23114173
- Zalas-Wiecek P, Michalska A, Grabczewska E, Olczak A, Pawlowska M, Gospodarek E. Human meningitis casued by Streptococcus suis - case report from Poland. J Med Microbiol 2013; 62:483 5; http://dx.doi.org/10.1099/jmm.0.046599-0; PMID: 23222864
- Rosenkranz M, Elsner HA, Stürenburg HJ, Weiller C, Röther J, Sobottka I. Streptococcus suis meningitis and septicemia contracted from a wild boar in Germany. J Neurol 2003; 250:869 - 70; http://dx.doi.org/10.1007/s00415-003-1103-3; PMID: 12883932
- Spiss HK, Kofler M, Hausdorfer H, Pfausler B, Schmutzhard E. [Streptococcus suis meningitis and neurophysiology of the acoustic system. First case report from Austria]. Nervenarzt 1999; 70:738 - 41; http://dx.doi.org/10.1007/s001150050503; PMID: 10483574
- Kopić J, Paradzik MT, Pandak N. Streptococcus suis infection as a cause of severe illness: 2 cases from Croatia. Scand J Infect Dis 2002; 34:683 - 4; http://dx.doi.org/10.1080/00365540210147769; PMID: 12374361
- Camporese A, Tizianel G, Bruschetta G, Cruciatti B, Pomes A. Human meningitis caused by Streptococcus suis: the first case report from north-eastern Italy. Infez Med 2007; 15:111 - 4; PMID: 17598998
- Manzin A, Palmieri C, Serra C, Saddi B, Princivalli MS, Loi G, Angioni G, Tiddia F, Varaldo PE, Facinelli B. Streptococcus suis meningitis without history of animal contact, Italy. Emerg Infect Dis 2008; 14:1946 - 8; http://dx.doi.org/10.3201/eid1412.080679; PMID: 19046529
- Princivalli MS, Palmieri C, Magi G, Vignaroli C, Manzin A, Camporese A, Barocci S, Magistrali C, Facinelli B. Genetic diversity of Streptococcus suis clinical isolates from pigs and humans in Italy (2003-2007). Euro Surveill 2009; 14:14; PMID: 19712640
- Mazokopakis EE, Kofteridis DP, Papadakis JA, Gikas AH, Samonis GJ. First case report of Streptococcus suis septicaemia and meningitis from Greece. Eur J Neurol 2005; 12:487 - 9; http://dx.doi.org/10.1111/j.1468-1331.2005.00998.x; PMID: 15885057
- Tambyah PA, Kumarasinghe G, Chan HL, Lee KO. Streptococcus suis infection complicated by purpura fulminans and rhabdomyolysis: case report and review. Clin Infect Dis 1997; 24:710 - 2; http://dx.doi.org/10.1093/clind/24.4.710; PMID: 9145747
- Shneerson JM, Chattopadhyay B, Murphy MF, Fawcett IW. Permanent perceptive deafness due to Streptococcus suis type II infection. J Laryngol Otol 1980; 94:425 - 7; http://dx.doi.org/10.1017/S0022215100089040; PMID: 7391667
- Huh HJ, Park KJ, Jang JH, Lee M, Lee JH, Ahn YH, Kang CI, Ki CS, Lee NY. Streptococcus suis meningitis with bilateral sensorineural hearing loss. Korean J Lab Med 2011; 31:205 - 11; http://dx.doi.org/10.3343/kjlm.2011.31.3.205; PMID: 21779197
- Kim H, Lee SH, Moon HW, Kim JY, Lee SH, Hur M, Yun YM. Streptococcus suis causes septic arthritis and bacteremia: phenotypic characterization and molecular confirmation. Korean J Lab Med 2011; 31:115 - 7; http://dx.doi.org/10.3343/kjlm.2011.31.2.115; PMID: 21474987
- Oh YJ, Song SH. A case of Streptococcus suis infection causing pneumonia with empyema in Korea. Tuberc Respir Dis (Seoul) 2012; 73:178 - 81; http://dx.doi.org/10.4046/trd.2012.73.3.178; PMID: 23166552
- Ibaraki M, Fujita N, Tada M, Ohtaki O, Nagai H. [A Japanese case of Streptococcus suis meningitis associated with lumbar epidural abscess]. Rinsho Shinkeigaku 2003; 43:176 - 9; PMID: 12884827
- Ma E, Chung PH, So T, Wong L, Choi KM, Cheung DT, Kam KM, Chuang SK, Tsang T, Collaborative Study Group on Streptococcus suis infection in Hong Kong. Streptococcus suis infection in Hong Kong: an emerging infectious disease?. Epidemiol Infect 2008; 136:1691 - 7; http://dx.doi.org/10.1017/S0950268808000332; PMID: 18252026
- Ip M, Fung KS, Chi F, Cheuk ES, Chau SS, Wong BW, Lui S, Hui M, Lai RW, Chan PK. Streptococcus suis in Hong Kong. Diagn Microbiol Infect Dis 2007; 57:15 - 20; http://dx.doi.org/10.1016/j.diagmicrobio.2006.05.011; PMID: 16860513
- Hui AC, Ng KC, Tong PY, Mok V, Chow KM, Wu A, Wong LK. Bacterial meningitis in Hong Kong: 10-years’ experience. Clin Neurol Neurosurg 2005; 107:366 - 70; http://dx.doi.org/10.1016/j.clineuro.2004.10.006; PMID: 16023529
- Kay R, Cheng AF, Tse CY. Streptococcus suis infection in Hong Kong. QJM 1995; 88:39 - 47; PMID: 7894987
- Woo J. Streptococcus suis meningitis in man in Hong Kong. Trans R Soc Trop Med Hyg 1986; 80:848 - 9; http://dx.doi.org/10.1016/0035-9203(86)90403-7; PMID: 3603627
- Chau PY, Huang CY, Kay R. Streptococcus suis meningitis. An important underdiagnosed disease in Hong Kong. Med J Aust 1983; 1:414 - 6, 417; PMID: 6835158
- Tsai HY, Liao CH, Liu CY, Huang YT, Teng LJ, Hsueh PR. Streptococcus suis infection in Taiwan, 2000-2011. Diagn Microbiol Infect Dis 2012; 74:75 - 7; http://dx.doi.org/10.1016/j.diagmicrobio.2012.05.013; PMID: 22705228
- Yen MY, Liu YC, Wang JH, Chen YS, Wang YH, Cheng DL. Streptococcus suis meningitis complicated with permanent perceptive deafness: report of a case. J Formos Med Assoc 1994; 93:349 - 51; PMID: 7914782
- Kennedy KJ, Jadeer AA, Ong CW, Senanayake SN, Collignon PJ. Two cases of Streptococcus suis endocarditis in Australian piggery workers. Med J Aust 2008; 189:413; PMID: 18837692
- Tramontana AR, Graham M, Sinickas V, Bak N. An Australian case of Streptococcus suis toxic shock syndrome associated with occupational exposure to animal carcasses. Med J Aust 2008; 188:538 - 9; PMID: 18459929
- Robertson ID. Streptococcus suis type 2--a zoonotic agent in New Zealand. N Z Med J 1986; 99:167 - 8; PMID: 3457300
- Dickie AS, Bremner DA, Wong PY, North JD, Robertson ID. Streptococcus suis bacteraemia. N Z Med J 1987; 100:677 - 8; PMID: 3452149
- Teekakirikul P, Wiwanitkit V. Streptococcus suis infection: overview of case reports in Thailand. Southeast Asian J Trop Med Public Health 2003; 34:Suppl 2 178 - 83; PMID: 19230589
- Ibaraki M, Fujita N, Tada M, Ohtaki O, Nagai H. [A Japanese case of Streptococcus suis meningitis associated with lumbar epidural abscess]. Rinsho Shinkeigaku 2003; 43:176 - 9; PMID: 12884827
- Fittipaldi N, Collis T, Prothero B, Gottschalk M. Streptococcus suis meningitis, Hawaii. Emerg Infect Dis 2009; 15:2067 - 9; http://dx.doi.org/10.3201/eid1512.090825; PMID: 19961708
- Smith TC, Capuano AW, Boese B, Myers KP, Gray GC. Exposure to Streptococcus suis among US swine workers. Emerg Infect Dis 2008; 14:1925 - 7; http://dx.doi.org/10.3201/eid1412.080162; PMID: 19046523
- Donsakul K, Dejthevaporn C, Witoonpanich R. Streptococcus suis infection: clinical features and diagnostic pitfalls. Southeast Asian J Trop Med Public Health 2003; 34:154 - 8; PMID: 12971528
- Heidt MC, Mohamed W, Hain T, Vogt PR, Chakraborty T, Domann E. Human infective endocarditis caused by Streptococcus suis serotype 2. J Clin Microbiol 2005; 43:4898 - 901; http://dx.doi.org/10.1128/JCM.43.9.4898-4901.2005; PMID: 16145171
- Schmid S, O’Connor M, Okwumabua O. The pathogenicity island-like DNA segment associated with Chinese outbreak strain of Streptococcus suis serotype 2 is absent in the United States isolates. Int J Mol Epidemiol Genet 2011; 2:56 - 60; PMID: 21537402
- Ye C, Zheng H, Zhang J, Jing H, Wang L, Xiong Y, Wang W, Zhou Z, Sun Q, Luo X, et al. Clinical, experimental, and genomic differences between intermediately pathogenic, highly pathogenic, and epidemic Streptococcus suis.. J Infect Dis 2009; 199:97 - 107; http://dx.doi.org/10.1086/594370; PMID: 19016627
- Wang C, Zheng F, Pan X, Dong R, Hu D, Qin Y, Tang J.. Identification of a virulent Streptococcus suis serotype 2 strain 07NJH06 isolated from patient with streptococcal encephalomeningitis syndrome. Journal of pathogen biology 2009; 4:161 5
- Feng Y, Pan X, Sun W, Wang C, Zhang H, Li X, Ma Y, Shao Z, Ge J, Zheng F, et al. Streptococcus suis enolase functions as a protective antigen displayed on the bacterial cell surface. J Infect Dis 2009; 200:1583 - 92; http://dx.doi.org/10.1086/644602; PMID: 19848587
- Ju Y, Hao HJ, Xiong GH, Geng HR, Zheng YL, Wang J, Cao Y, Yang YH, Cai XH, Jiang YQ. Development of colloidal gold-based immunochromatographic assay for rapid detection of Streptococcus suis serotype 2. Vet Immunol Immunopathol 2010; 133:207 - 11; http://dx.doi.org/10.1016/j.vetimm.2009.08.010; PMID: 19733402
- Yang J, Jin M, Chen J, Yang Y, Zheng P, Zhang A, Song Y, Zhou H, Chen H. Development and evaluation of an immunochromatographic strip for detection of Streptococcus suis type 2 antibody. J Vet Diagn Invest 2007; 19:355 - 61; http://dx.doi.org/10.1177/104063870701900403; PMID: 17609343
- Wang H, Yuan R, Chai Y, Cao Y, Gan X, Chen Y, Wang Y. An ultrasensitive peroxydisulfate electrochemiluminescence immunosensor for Streptococcus suis serotype 2 based on L-cysteine combined with mimicking bi-enzyme synergetic catalysis to in situ generate coreactant. Biosens Bioelectron 2013; 43:63 - 8; http://dx.doi.org/10.1016/j.bios.2012.11.038; PMID: 23277341
- Wilson TL, Jeffers J, Rapp-Gabrielson VJ, Martin S, Klein LK, Lowery DE, Fuller TE. A novel signature-tagged mutagenesis system for Streptococcus suis serotype 2. Vet Microbiol 2007; 122:135 - 45; http://dx.doi.org/10.1016/j.vetmic.2006.12.025; PMID: 17275218
- Segura M, Gottschalk M, Olivier M. Encapsulated Streptococcus suis inhibits activation of signaling pathways involved in phagocytosis. Infect Immun 2004; 72:5322 - 30; http://dx.doi.org/10.1128/IAI.72.9.5322-5330.2004; PMID: 15322029
- Vecht U, Wisselink HJ, Jellema ML, Smith HE. Identification of two proteins associated with virulence of Streptococcus suis type 2. Infect Immun 1991; 59:3156 - 62; PMID: 1879937
- de Greeff A, Buys H, Verhaar R, Dijkstra J, van Alphen L, Smith HE. Contribution of fibronectin-binding protein to pathogenesis of Streptococcus suis serotype 2. Infect Immun 2002; 70:1319 - 25; http://dx.doi.org/10.1128/IAI.70.3.1319-1325.2002; PMID: 11854216
- Okwumabua O, Chinnapapakkagari S. Identification of the gene encoding a 38-kilodalton immunogenic and protective antigen of Streptococcus suis.. Clin Diagn Lab Immunol 2005; 12:484 - 90; PMID: 15817754
- Lun S, Perez-Casal J, Connor W, Willson PJ. Role of suilysin in pathogenesis of Streptococcus suis capsular serotype 2. Microb Pathog 2003; 34:27 - 37; http://dx.doi.org/10.1016/S0882-4010(02)00192-4; PMID: 12620382
- Xu L, Huang B, Du H, Zhang XC, Xu J, Li X, Rao Z. Crystal structure of cytotoxin protein suilysin from Streptococcus suis.. Protein Cell 2010; 1:96 - 105; http://dx.doi.org/10.1007/s13238-010-0012-3; PMID: 21204001
- Takeuchi D, Akeda Y, Nakayama T, Kerdsin A, Sano Y, Kanda T, Hamada S, Dejsirilert S, Oishi K. The contribution of suilysin to the pathogenesis of Streptococcus suis meningitis. J Infect Dis 2014; forthcoming PMID: 24285845
- Bonifait L, de la Cruz Dominguez-Punaro M, Vaillancourt K, Bart C, Slater J, Frenette M, Gottschalk M, Grenier D. The cell envelope subtilisin-like proteinase is a virulence determinant for Streptococcus suis. BMC Microbiol 2010; 10:42; http://dx.doi.org/10.1186/1471-2180-10-42; PMID: 20146817
- Bonifait L, Vaillancourt K, Gottschalk M, Frenette M, Grenier D. Purification and characterization of the subtilisin-like protease of Streptococcus suis that contributes to its virulence. Vet Microbiol 2011; 148:333 - 40; http://dx.doi.org/10.1016/j.vetmic.2010.09.024; PMID: 21030165
- Shao Z, Pan X, Li X, Liu W, Han M, Wang C, Wang J, Zheng F, Cao M, Tang J. HtpS, a novel immunogenic cell surface-exposed protein of Streptococcus suis, confers protection in mice. FEMS Microbiol Lett 2011; 314:174 - 82; http://dx.doi.org/10.1111/j.1574-6968.2010.02162.x; PMID: 21133988
- Chen B, Zhang A, Li R, Mu X, He H, Chen H, Jin M. Evaluation of the protective efficacy of a newly identified immunogenic protein, HP0272, of Streptococcus suis. FEMS Microbiol Lett 2010; 307:12 - 8; http://dx.doi.org/10.1111/j.1574-6968.2010.01944.x; PMID: 20402782
- Mandanici F, Gómez-Gascón L, Garibaldi M, Olaya-Abril A, Luque I, Tarradas C, Mancuso G, Papasergi S, Bárcena JA, Teti G, et al. A surface protein of Streptococcus suis serotype 2 identified by proteomics protects mice against infection. J Proteomics 2010; 73:2365 - 9; http://dx.doi.org/10.1016/j.jprot.2010.07.009; PMID: 20656083
- Li W, Wan Y, Tao Z, Chen H, Zhou R. A novel fibronectin-binding protein of Streptococcus suis serotype 2 contributes to epithelial cell invasion and in vivo dissemination. Vet Microbiol 2013; 162:186 94; http://dx.doi.org/10.1016/j.vetmic.2012.09.004; PMID: 23021642
- Hu P, Yang M, Zhang A, Wu J, Chen B, Hua Y, Yu J, Xiao J, Jin M. Complete genome sequence of Streptococcus suis serotype 14 strain JS14. J Bacteriol 2011; 193:2375 - 6; http://dx.doi.org/10.1128/JB.00083-11; PMID: 21398551
- Zhang A, Chen B, Li R, Mu X, Han L, Zhou H, Chen H, Meilin J. Identification of a surface protective antigen, HP0197 of Streptococcus suis serotype 2. Vaccine 2009; 27:5209 - 13; http://dx.doi.org/10.1016/j.vaccine.2009.06.074; PMID: 19596417
- Yuan ZZ, Yan XJ, Zhang AD, Chen B, Shen YQ, Jin ML. The molecular mechanism by which surface antigen HP0197 mediates host cell attachment in the pathogenic bacteria Streptococcus suis.. J Biol Chem 2013; 288:956 63; http://dx.doi.org/10.1074/jbc.M112.388686; PMID: 23184929
- Zhang A, Chen B, Yuan Z, Li R, Liu C, Zhou H, Chen H, Jin M. HP0197 contributes to CPS synthesis and the virulence of Streptococcus suis via CcpA. PLoS One 2012; 7:e50987; http://dx.doi.org/10.1371/journal.pone.0050987; PMID: 23226442
- Willenborg J, Fulde M, de Greeff A, Rohde M, Smith HE, Valentin-Weigand P, Goethe R. Role of glucose and CcpA in capsule expression and virulence of Streptococcus suis.. Microbiology 2011; 157:1823 - 33; http://dx.doi.org/10.1099/mic.0.046417-0; PMID: 21349980
- Tang Y, Wu W, Zhang X, Lu Z, Chen J, Fang W. Catabolite control protein A of Streptococcus suis type 2 contributes to sugar metabolism and virulence. J Microbiol 2012; 50:994 - 1002; http://dx.doi.org/10.1007/s12275-012-2035-3; PMID: 23274986
- Baums CG, Kaim U, Fulde M, Ramachandran G, Goethe R, Valentin-Weigand P. Identification of a novel virulence determinant with serum opacification activity in Streptococcus suis.. Infect Immun 2006; 74:6154 - 62; http://dx.doi.org/10.1128/IAI.00359-06; PMID: 17057090
- Li Y, Martinez G, Gottschalk M, Lacouture S, Willson P, Dubreuil JD, Jacques M, Harel J. Identification of a surface protein of Streptococcus suis and evaluation of its immunogenic and protective capacity in pigs. Infect Immun 2006; 74:305 - 12; http://dx.doi.org/10.1128/IAI.74.1.305-312.2006; PMID: 16368985
- Li Y, Gottschalk M, Esgleas M, Lacouture S, Dubreuil JD, Willson P, Harel J. Immunization with recombinant Sao protein confers protection against Streptococcus suis infection. Clin Vaccine Immunol 2007; 14:937 - 43; http://dx.doi.org/10.1128/CVI.00046-07; PMID: 17567767
- Roy D, Fittipaldi N, Dumesnil A, Lacouture S, Gottschalk M. The protective protein Sao (surface antigen one) is not a critical virulence factor for Streptococcus suis serotype 2. Microb Pathog 2014; 67-68C:31 35; http://dx.doi.org/10.1016/j.micpath.2014.02.002; PMID: 24530923
- Zhang H, Fan H, Lu C. Identification of a novel virulence-related gene in Streptococcus suis type 2 strains. Curr Microbiol 2010; 61:494 - 9; http://dx.doi.org/10.1007/s00284-010-9643-0; PMID: 20386910
- Garibaldi M, Rodríguez-Ortega MJ, Mandanici F, Cardaci A, Midiri A, Papasergi S, Gambadoro O, Cavallari V, Teti G, Beninati C. Immunoprotective activities of a Streptococcus suis pilus subunit in murine models of infection. Vaccine 2010; 28:3609 - 16; http://dx.doi.org/10.1016/j.vaccine.2010.01.009; PMID: 20079873
- Aranda J, Teixidó L, Fittipaldi N, Cortés P, Llagostera M, Gottschalk M, Barbé J. Inactivation of the gene encoding zinc-binding lipoprotein 103 impairs the infectivity of Streptococcus suis.. Can J Vet Res 2012; 76:72 - 6; PMID: 22754099
- Si Y, Yuan F, Chang H, Liu X, Li H, Cai K, Xu Z, Huang Q, Bei W, Chen H. Contribution of glutamine synthetase to the virulence of Streptococcus suis serotype 2. Vet Microbiol 2009; 139:80 - 8; http://dx.doi.org/10.1016/j.vetmic.2009.04.024; PMID: 19447571
- Okwumabua O, Persaud JS, Reddy PG. Cloning and characterization of the gene encoding the glutamate dehydrogenase of Streptococcus suis serotype 2. Clin Diagn Lab Immunol 2001; 8:251 - 7; PMID: 11238204
- Zhang A, Chen B, Mu X, Li R, Zheng P, Zhao Y, Chen H, Jin M. Identification and characterization of a novel protective antigen, Enolase of Streptococcus suis serotype 2. Vaccine 2009; 27:1348 - 53; http://dx.doi.org/10.1016/j.vaccine.2008.12.047; PMID: 19150475
- Esgleas M, Dominguez-Punaro MdeL, Li Y, Harel J, Dubreuil JD, Gottschalk M. Immunization with SsEno fails to protect mice against challenge with Streptococcus suis serotype 2. FEMS Microbiol Lett 2009; 294:82 - 8; http://dx.doi.org/10.1111/j.1574-6968.2009.01551.x; PMID: 19493012
- Zhang XH, He KW, Duan ZT, Zhou JM, Yu ZY, Ni YX, Lu CP. Identification and characterization of inosine 5-monophosphate dehydrogenase in Streptococcus suis type 2. Microb Pathog 2009; 47:267 - 73; http://dx.doi.org/10.1016/j.micpath.2009.09.001; PMID: 19744553
- Fittipaldi N, Sekizaki T, Takamatsu D, Harel J, Domínguez-Punaro MdeL, Von Aulock S, Draing C, Marois C, Kobisch M, Gottschalk M. D-alanylation of lipoteichoic acid contributes to the virulence of Streptococcus suis.. Infect Immun 2008; 76:3587 - 94; http://dx.doi.org/10.1128/IAI.01568-07; PMID: 18474639
- Fittipaldi N, Sekizaki T, Takamatsu D, de la Cruz Domínguez-Punaro M, Harel J, Bui NK, Vollmer W, Gottschalk M. Significant contribution of the pgdA gene to the virulence of Streptococcus suis.. Mol Microbiol 2008; 70:1120 - 35; http://dx.doi.org/10.1111/j.1365-2958.2008.06463.x; PMID: 18990186
- Ferrando ML, Fuentes S, de Greeff A, Smith H, Wells JM. ApuA, a multifunctional alpha-glucan-degrading enzyme of Streptococcus suis, mediates adhesion to porcine epithelium and mucus. Microbiology 2010; 156:2818 - 28; http://dx.doi.org/10.1099/mic.0.037960-0; PMID: 20522493
- Ge J, Feng Y, Ji H, Zhang H, Zheng F, Wang C, Yin Z, Pan X, Tang J. Inactivation of dipeptidyl peptidase IV attenuates the virulence of Streptococcus suis serotype 2 that causes streptococcal toxic shock syndrome. Curr Microbiol 2009; 59:248 - 55; http://dx.doi.org/10.1007/s00284-009-9425-8; PMID: 19484301
- Wang C, Li M, Feng Y, Zheng F, Dong Y, Pan X, Cheng G, Dong R, Hu D, Feng X, et al. The involvement of sortase A in high virulence of STSS-causing Streptococcus suis serotype 2. Arch Microbiol 2009; 191:23 - 33; http://dx.doi.org/10.1007/s00203-008-0425-z; PMID: 18716756
- Vanier G, Sekizaki T, Domínguez-Punaro MC, Esgleas M, Osaki M, Takamatsu D, Segura M, Gottschalk M. Disruption of srtA gene in Streptococcus suis results in decreased interactions with endothelial cells and extracellular matrix proteins. Vet Microbiol 2008; 127:417 - 24; http://dx.doi.org/10.1016/j.vetmic.2007.08.032; PMID: 17954016
- Zhang A, Mu X, Chen B, Han L, Chen H, Jin M. IgA1 protease contributes to the virulence of Streptococcus suis.. Vet Microbiol 2011; 148:436 - 9; http://dx.doi.org/10.1016/j.vetmic.2010.09.027; PMID: 21041043
- Wang Y, Zhang W, Wu Z, Zhu X, Lu C. Functional analysis of luxS in Streptococcus suis reveals a key role in biofilm formation and virulence. Vet Microbiol 2011; 152:151 - 60; http://dx.doi.org/10.1016/j.vetmic.2011.04.029; PMID: 21621932
- Cao M, Feng Y, Wang C, Zheng F, Li M, Liao H, Mao Y, Pan X, Wang J, Hu D, et al. Functional definition of LuxS, an autoinducer-2 (AI-2) synthase and its role in full virulence of Streptococcus suis serotype 2. J Microbiol 2011; 49:1000 - 11; http://dx.doi.org/10.1007/s12275-011-1523-1; PMID: 22203565
- Feng Y, Cao M, Shi J, Zhang H, Hu D, Zhu J, Zhang X, Geng M, Zheng F, Pan X, et al. Attenuation of Streptococcus suis virulence by the alteration of bacterial surface architecture. Sci Rep 2012; 2:710; http://dx.doi.org/10.1038/srep00710; PMID: 23050094
- Lecours MP, Fittipaldi N, Takamatsu D, Okura M, Segura M, Goyette-Desjardins G, Van Calsteren MR, Gottschalk M. Sialylation of Streptococcus suis serotype 2 is essential for capsule expression but is not responsible for the main capsular epitope. Microbes Infect 2012; 14:941 - 50; http://dx.doi.org/10.1016/j.micinf.2012.03.008; PMID: 22521569
- Esgleas M, Li Y, Hancock MA, Harel J, Dubreuil JD, Gottschalk M. Isolation and characterization of alpha-enolase, a novel fibronectin-binding protein from Streptococcus suis.. Microbiology 2008; 154:2668 - 79; http://dx.doi.org/10.1099/mic.0.2008/017145-0; PMID: 18757800
- Lu Q, Lu H, Qi J, Lu G, Gao GF. An octamer of enolase from Streptococcus suis.. Protein Cell 2012; 3:769 - 80; http://dx.doi.org/10.1007/s13238-012-2040-7; PMID: 23055041
- Chen H, Liao H, Wang C, Pan X, Tang J. [Construction and in vitro assay of the sortase BCD geneknock-out mutant of Streptococcus suis 2]. Wei Sheng Wu Xue Bao 2011; 51:386 - 92; PMID: 21604553
- Gruening P, Fulde M, Valentin-Weigand P, Goethe R. Structure, regulation, and putative function of the arginine deiminase system of Streptococcus suis.. J Bacteriol 2006; 188:361 - 9; http://dx.doi.org/10.1128/JB.188.2.361-369.2006; PMID: 16385025
- Liu P, Pian Y, Li X, Liu R, Xie W, Zhang C, Zheng Y, Jiang Y, Yuan Y. Streptococcus suis adenosine synthase functions as an effector in evasion of PMNs-mediated innate immunity. J Infect Dis 2014; forthcoming http://dx.doi.org/10.1093/infdis/jiu050; PMID: 24446521
- Goble AM, Feng Y, Raushel FM, Cronan JE. Discovery of a cAMP deaminase that quenches cyclic AMP-dependent regulation. ACS Chem Biol 2013; 8:2622 - 9; http://dx.doi.org/10.1021/cb4004628; PMID: 24074367
- Aranda J, Garrido ME, Fittipaldi N, Cortés P, Llagostera M, Gottschalk M, Barbé J. The cation-uptake regulators AdcR and Fur are necessary for full virulence of Streptococcus suis.. Vet Microbiol 2010; 144:246 - 9; http://dx.doi.org/10.1016/j.vetmic.2009.12.037; PMID: 20133089
- Zhang T, Ding Y, Li T, Wan Y, Li W, Chen H, Zhou R. A Fur-like protein PerR regulates two oxidative stress response related operons dpr and metQIN in Streptococcus suis.. BMC Microbiol 2012; 12:85; http://dx.doi.org/10.1186/1471-2180-12-85; PMID: 22646062
- Wang J, Gao Y, Teng K, Zhang J, Sun S, Zhong J. Restoration of bioactive lantibiotic suicin from a remnant lan locus of pathogenic Streptococcus suis serotype 2. Appl Environ Microbiol 2014; 80:1062 - 71; http://dx.doi.org/10.1128/AEM.03213-13; PMID: 24271178
- Han H, Liu C, Wang Q, Xuan C, Zheng B, Tang J, Yan J, Zhang J, Li M, Cheng H, et al. The two-component system Ihk/Irr contributes to the virulence of Streptococcus suis serotype 2 strain 05ZYH33 through alteration of the bacterial cell metabolism. Microbiology 2012; 158:1852 - 66; http://dx.doi.org/10.1099/mic.0.057448-0; PMID: 22504441
- Wang H, Shen X, Zhao Y, Wang M, Zhong Q, Chen T, Hu F, Li M. Identification and proteome analysis of the two-component VirR/VirS system in epidemic Streptococcus suis serotype 2. FEMS Microbiol Lett 2012; 333:160 - 8; http://dx.doi.org/10.1111/j.1574-6968.2012.02611.x; PMID: 22670712
- Xu J, Fu S, Liu M, Xu Q, Bei W, Chen H, Tan C. The two-component system NisK/NisR contributes to the virulence of Streptococcus suis serotype 2. Microbiol Res 2013; forthcoming http://dx.doi.org/10.1016/j.micres.2013.11.002; PMID: 24342108
- Wu T, Chang H, Tan C, Bei W, Chen H. The orphan response regulator RevSC21 controls the attachment of Streptococcus suis serotype-2 to human laryngeal epithelial cells and the expression of virulence genes. FEMS Microbiol Lett 2009; 292:170 - 81; http://dx.doi.org/10.1111/j.1574-6968.2008.01486.x; PMID: 19210676
- de Greeff A, Buys H, van Alphen L, Smith HE. Response regulator important in pathogenesis of Streptococcus suis serotype 2. Microb Pathog 2002; 33:185 - 92; PMID: 12385746
- Ju AP, Wang CJ, Li M, Cheng G, Zheng F, Pan XZ, Lu CP, Tang JQ. [Construction of RevS gene knock-out mutant of Streptococcus suis serotype 2]. Zhonghua Liu Xing Bing Xue Za Zhi 2008; 29:59 - 64; PMID: 18785481
- Shen X, Zhong Q, Zhao Y, Yin S, Chen T, Hu F, Li M. Proteome analysis of the two-component SalK/SalR system in Epidemic Streptococcus suis serotype 2. Curr Microbiol 2013; 67:118 - 22; http://dx.doi.org/10.1007/s00284-013-0343-4; PMID: 23463517
- Wu T, Zhao Z, Zhang L, Ma H, Lu K, Ren W, Liu Z, Chang H, Bei W, Qiu Y, et al. Trigger factor of Streptococcus suis is involved in stress tolerance and virulence. Microb Pathog 2011; 51:69 - 76; http://dx.doi.org/10.1016/j.micpath.2010.10.001; PMID: 21093574
- Zhao Y, Liu G, Li S, Wang M, Song J, Wang J, Tang J, Li M, Hu F. Role of a type IV-like secretion system of Streptococcus suis 2 in the development of streptococcal toxic shock syndrome. J Infect Dis 2011; 204:274 - 81; http://dx.doi.org/10.1093/infdis/jir261; PMID: 21673039
- Vadeboncoeur N, Segura M, Al-Numani D, Vanier G, Gottschalk M. Pro-inflammatory cytokine and chemokine release by human brain microvascular endothelial cells stimulated by Streptococcus suis serotype 2. FEMS Immunol Med Microbiol 2003; 35:49 - 58; http://dx.doi.org/10.1111/j.1574-695X.2003.tb00648.x; PMID: 12589957
- Domínguez-Punaro MC, Segura M, Plante MM, Lacouture S, Rivest S, Gottschalk M. Streptococcus suis serotype 2, an important swine and human pathogen, induces strong systemic and cerebral inflammatory responses in a mouse model of infection. J Immunol 2007; 179:1842 - 54; PMID: 17641051
- Schwerk C, Adam R, Borkowski J, Schneider H, Klenk M, Zink S, Quednau N, Schmidt N, Stump C, Sagar A, et al. In vitro transcriptome analysis of porcine choroid plexus epithelial cells in response to Streptococcus suis: release of pro-inflammatory cytokines and chemokines. Microbes Infect 2011; 13:953 - 62; http://dx.doi.org/10.1016/j.micinf.2011.05.012; PMID: 21683799
- Zheng H, Punaro MC, Segura M, Lachance C, Rivest S, Xu J, Houde M, Gottschalk M. Toll-like receptor 2 is partially involved in the activation of murine astrocytes by Streptococcus suis, an important zoonotic agent of meningitis. J Neuroimmunol 2011; 234:71 - 83; http://dx.doi.org/10.1016/j.jneuroim.2011.02.005; PMID: 21429596
- Graveline R, Segura M, Radzioch D, Gottschalk M. TLR2-dependent recognition of Streptococcus suis is modulated by the presence of capsular polysaccharide which modifies macrophage responsiveness. Int Immunol 2007; 19:375 - 89; http://dx.doi.org/10.1093/intimm/dxm003; PMID: 17307800
- de Greeff A, Benga L, Wichgers Schreur PJ, Valentin-Weigand P, Rebel JM, Smith HE. Involvement of NF-kappaB and MAP-kinases in the transcriptional response of alveolar macrophages to Streptococcus suis.. Vet Microbiol 2010; 141:59 - 67; http://dx.doi.org/10.1016/j.vetmic.2009.07.031; PMID: 19709818
- Wu T, Yuan F, Chang H, Zhang L, Chen G, Tan C, Chen H, Bei W. Identification of a novel angiogenin inhibitor 1 and its association with hyaluronidase of Streptococcus suis serotype 2. Microb Pathog 2010; 49:32 - 7; http://dx.doi.org/10.1016/j.micpath.2010.03.002; PMID: 20307645
- Wisselink HJ, Stockhofe-Zurwieden N, Hilgers LA, Smith HE. Assessment of protective efficacy of live and killed vaccines based on a non-encapsulated mutant of Streptococcus suis serotype 2. Vet Microbiol 2002; 84:155 - 68; http://dx.doi.org/10.1016/S0378-1135(01)00452-7; PMID: 11731168
- Busque P, Higgins R, Caya F, Quessy S. Immunization of pigs against Streptococcus suis serotype 2 infection using a live avirulent strain. Can J Vet Res 1997; 61:275 - 9; PMID: 9342451
- Andresen LO, Tegtmeier C. Passive immunization of pigs against experimental infection with Streptococcus suis serotype 2. Vet Microbiol 2001; 81:331 - 44; http://dx.doi.org/10.1016/S0378-1135(01)00359-5; PMID: 11390114
- Wisselink HJ, Vecht U, Stockhofe-Zurwieden N, Smith HE. Protection of pigs against challenge with virulent Streptococcus suis serotype 2 strains by a muramidase-released protein and extracellular factor vaccine. Vet Rec 2001; 148:473 - 7; http://dx.doi.org/10.1136/vr.148.15.473; PMID: 11334073
- Liu L, Cheng G, Wang C, Pan X, Cong Y, Pan Q, Wang J, Zheng F, Hu F, Tang J. Identification and experimental verification of protective antigens against Streptococcus suis serotype 2 based on genome sequence analysis. Curr Microbiol 2009; 58:11 - 7; http://dx.doi.org/10.1007/s00284-008-9258-x; PMID: 18839251
- Li J, Xia J, Tan C, Zhou Y, Wang Y, Zheng C, Chen H, Bei W. Evaluation of the immunogenicity and the protective efficacy of a novel identified immunogenic protein, SsPepO, of Streptococcus suis serotype 2. Vaccine 2011; 29:6514 - 9; http://dx.doi.org/10.1016/j.vaccine.2011.07.010; PMID: 21767591
- Chen B, Zhang A, Li R, Mu X, He H, Chen H, Jin M. Evaluation of the protective efficacy of a newly identified immunogenic protein, HP0272, of Streptococcus suis.. FEMS Microbiol Lett 2010; 307:12 - 8; http://dx.doi.org/10.1111/j.1574-6968.2010.01944.x; PMID: 20402782
- Dragojlović J, Milosević B, Sasić N, Pelemis M, Sasić M. [Streptococcus suis infection--clinical manifestations]. Med Pregl 2005; 58:236 - 9; http://dx.doi.org/10.2298/MPNS0506236D; PMID: 16526227
- Hoa NT, Chieu TT, Nghia HD, Mai NT, Anh PH, Wolbers M, Baker S, Campbell JI, Chau NV, Hien TT, et al. The antimicrobial resistance patterns and associated determinants in Streptococcus suis isolated from humans in southern Vietnam, 1997-2008. BMC Infect Dis 2011; 11:6; http://dx.doi.org/10.1186/1471-2334-11-6; PMID: 21208459
- Nghia HD, Tu TP, Wolbers M, Thai CQ, Hoang NV, Nga TV, Thao TP, Phu NH, Chau TT, Sinh DX, et al. Risk factors of Streptococcus suis infection in Vietnam. A case-control study. PLoS One 2011; 6:e17604; http://dx.doi.org/10.1371/journal.pone.0017604; PMID: 21408132
- Ngo TH, Tran TB, Tran TT, Nguyen VD, Campbell J, Pham HA, Huynh HT, Nguyen VV, Bryant JE, Tran TH, et al. Slaughterhouse pigs are a major reservoir of Streptococcus suis serotype 2 capable of causing human infection in southern Vietnam. PLoS One 2011; 6:e17943; http://dx.doi.org/10.1371/journal.pone.0017943; PMID: 21464930
- Coolen L, Dens J, Baeck E, Claes C, Lins RL, Verbraeken H, Daelemans R. Streptococcus suis meningitis, permanent perceptive deafness and endophthalmitis. Intensive Care Med 1989; 15:545; PMID: 2607048
- Watkins EJ, Brooksby P, Schweiger MS, Enright SM. Septicaemia in a pig-farm worker. Lancet 2001; 357:38; http://dx.doi.org/10.1016/S0140-6736(00)03570-4; PMID: 11197360
- Arend SM, van Buchem MA, van Ogtrop ML, Thompson J. Septicaemia, meningitis and spondylodiscitis caused by Streptococcus suis type 2. Infection 1995; 23:128; http://dx.doi.org/10.1007/BF01833885; PMID: 7622264
- Maher D. Streptococcus suis septicaemia presenting as severe acute gastro-enteritis. J Infect 1991; 22:303 - 4; http://dx.doi.org/10.1016/S0163-4453(05)80022-2; PMID: 2071918
- Bergdoll MS, Crass BA, Reiser RF, Robbins RN, Davis JP. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. Lancet 1981; 1:1017 - 21; http://dx.doi.org/10.1016/S0140-6736(81)92186-3; PMID: 6112412
- Cohen ML, Falkow S. Protein antigens from Staphylococcus aureus strains associated with toxic-shock syndrome. Science 1981; 211:842 - 4; http://dx.doi.org/10.1126/science.7466361; PMID: 7466361
- Bassaris HP, Venezio FR, Morlock BA, Phair JP. Staphylococcus aureus in toxic-shock syndrome. J Infect Dis 1981; 144:386 - 7; http://dx.doi.org/10.1093/infdis/144.4.386; PMID: 7288219
- Schutzer SE, Fischetti VA, Zabriskie JB. Toxic shock syndrome and lysogeny in Staphylococcus aureus. Science 1983; 220:316 - 8; http://dx.doi.org/10.1126/science.6220467; PMID: 6220467
- Leelarasamee A, Nilakul C, Tien-Grim S, Srifuengfung S, Susaengrat W. Streptococcus suis toxic-shock syndrome and meningitis. J Med Assoc Thai 1997; 80:63 - 8; PMID: 9078819
- François B, Gissot V, Ploy MC, Vignon P. Recurrent septic shock due to Streptococcus suis. J Clin Microbiol 1998; 36:2395; PMID: 9675698
- Poggenborg R, Gaïni S, Kjaeldgaard P, Christensen JJ. Streptococcus suis: meningitis, spondylodiscitis and bacteraemia with a serotype 14 strain. Scand J Infect Dis 2008; 40:346 - 9; http://dx.doi.org/10.1080/00365540701716825; PMID: 18365920
- Feng Y, Zhang H. M. C, Wang C. Regulation of Virulence in Streptococcus suis.. J Bacteriol Parasitol 2012; 3:e108
- Zheng X, Zheng H, Lan R, Ye C, Wang Y, Zhang J, Jing H, Chen C, Segura M, Gottschalk M, et al. Identification of genes and genomic islands correlated with high pathogenicity in Streptococcus suis using whole genome tiling microarrays. PLoS One 2011; 6:e17987; http://dx.doi.org/10.1371/journal.pone.0017987; PMID: 21479213
- Wu Z, Li M, Wang C, Li J, Lu N, Zhang R, Jiang Y, Yang R, Liu C, Liao H, et al. Probing genomic diversity and evolution of Streptococcus suis serotype 2 by NimbleGen tiling arrays. BMC Genomics 2011; 12:219; http://dx.doi.org/10.1186/1471-2164-12-219; PMID: 21554741
- Kataoka Y, Sugimoto C, Nakazawa M, Kashiwazaki M. Detection of Streptococcus suis type 2 in tonsils of slaughtered pigs using improved selective and differential media. Vet Microbiol 1991; 28:335 - 42; http://dx.doi.org/10.1016/0378-1135(91)90068-Q; PMID: 1949547
- Okwumabua O, O’Connor M, Shull E. A polymerase chain reaction (PCR) assay specific for Streptococcus suis based on the gene encoding the glutamate dehydrogenase. FEMS Microbiol Lett 2003; 218:79 - 84; http://dx.doi.org/10.1111/j.1574-6968.2003.tb11501.x; PMID: 12583901
- Swildens B, Wisselink HJ, Engel B, Smith HE, Nielen M, Verheijden JH, Stegeman JA. Detection of extracellular factor-positive Streptococcus suis serotype 2 strains in tonsillar swabs of live sows by PCR. Vet Microbiol 2005; 109:223 - 8; http://dx.doi.org/10.1016/j.vetmic.2005.04.024; PMID: 16029935
- Kang I, Kim D, Han K, Seo HW, Oh Y, Park C, Lee J, Gottschalk M, Chae C. Optimized protocol for multiplex nested polymerase chain reaction to detect and differentiate Haemophilus parasuis, Streptococcus suis, and Mycoplasma hyorhinis in formalin-fixed, paraffin-embedded tissues from pigs with polyserositis. Can J Vet Res 2012; 76:195 - 200; PMID: 23277698
- Huy NT, Hang TT, Boamah D, Lan NT, Van Thanh P, Watanabe K, Huong VT, Kikuchi M, Ariyoshi K, Morita K, et al. Development of a single-tube loop-mediated isothermal amplification assay for detection of four pathogens of bacterial meningitis. FEMS Microbiol Lett 2012; 337:25 - 30; http://dx.doi.org/10.1111/1574-6968.12002; PMID: 22946506
- Zhang J, Zhu J, Ren H, Zhu S, Zhao P, Zhang F, Lv H, Hu D, Hao L, Geng M, et al. Rapid visual detection of highly pathogenic Streptococcus suis serotype 2 isolates by use of loop-mediated isothermal amplification. J Clin Microbiol 2013; 51:3250 - 6; http://dx.doi.org/10.1128/JCM.01183-13; PMID: 23884995
- Nga TV, Nghia HD, Tu TP, Diep TS, Mai NT, Chau TT, Sinh DX, Phu NH, Nga TT, Chau NV, et al. Real-time PCR for detection of Streptococcus suis serotype 2 in cerebrospinal fluid of human patients with meningitis. Diagn Microbiol Infect Dis 2011; 70:461 - 7; http://dx.doi.org/10.1016/j.diagmicrobio.2010.12.015; PMID: 21767702
- Yang W, Cai X, Hao Y, Liu Y, Wang S, Xing R, Gu J, Li C, Yue X, Yuan C, et al. Characterization of Streptococcus suis serotype 2 blood infections using RT-qPCR to quantify glutamate dehydrogenase copy numbers. J Microbiol Methods 2010; 83:326 - 9; http://dx.doi.org/10.1016/j.mimet.2010.09.013; PMID: 20869401
- Wisselink HJ, Reek FH, Vecht U, Stockhofe-Zurwieden N, Smits MA, Smith HE. Detection of virulent strains of Streptococcus suis type 2 and highly virulent strains of Streptococcus suis type 1 in tonsillar specimens of pigs by PCR. Vet Microbiol 1999; 67:143 - 57; http://dx.doi.org/10.1016/S0378-1135(99)00036-X; PMID: 10414368
- Gottschalk M, Lebrun A, Wisselink H, Dubreuil JD, Smith H, Vecht U. Production of virulence-related proteins by Canadian strains of Streptococcus suis capsular type 2. Can J Vet Res 1998; 62:75 - 9; PMID: 9442945
- Wisselink HJ, Joosten JJ, Smith HE. Multiplex PCR assays for simultaneous detection of six major serotypes and two virulence-associated phenotypes of Streptococcus suis in tonsillar specimens from pigs. J Clin Microbiol 2002; 40:2922 - 9; http://dx.doi.org/10.1128/JCM.40.8.2922-2929.2002; PMID: 12149353
- Berthelot-Hérault F, Morvan H, Kéribin AM, Gottschalk M, Kobisch M. Production of muraminidase-released protein (MRP), extracellular factor (EF) and suilysin by field isolates of Streptococcus suis capsular types 2, 1/2, 9, 7 and 3 isolated from swine in France. Vet Res 2000; 31:473 - 9; http://dx.doi.org/10.1051/vetres:2000133; PMID: 11050742
- Martinez G, Pestana de Castro AF, Ribeiro Pagnani KJ, Nakazato G, Dias da Silveira W, Gottschalk M. Clonal distribution of an atypical MRP+, EF*, and suilysin+ phenotype of virulent Streptococcus suis serotype 2 strains in Brazil. Can J Vet Res 2003; 67:52 - 5; PMID: 12528829
- Silva LM, Baums CG, Rehm T, Wisselink HJ, Goethe R, Valentin-Weigand P. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet Microbiol 2006; 115:117 - 27; http://dx.doi.org/10.1016/j.vetmic.2005.12.013; PMID: 16431041
- Liu Z, Zheng H, Gottschalk M, Bai X, Lan R, Ji S, Liu H, Xu J. Development of multiplex PCR assays for the identification of the 33 serotypes of Streptococcus suis.. PLoS One 2013; 8:e72070; http://dx.doi.org/10.1371/journal.pone.0072070; PMID: 23951285
- Marois C, Bougeard S, Gottschalk M, Kobisch M. Multiplex PCR assay for detection of Streptococcus suis species and serotypes 2 and 1/2 in tonsils of live and dead pigs. J Clin Microbiol 2004; 42:3169 - 75; http://dx.doi.org/10.1128/JCM.42.7.3169-3175.2004; PMID: 15243078
- Okwumabua O, Staats J, Chengappa MM. Detection of genomic heterogeneity in Streptococcus suis isolates by DNA restriction fragment length polymorphisms of rRNA genes (ribotyping). J Clin Microbiol 1995; 33:968 - 72; PMID: 7540630
- Marois C, Le Devendec L, Gottschalk M, Kobisch M. Detection and molecular typing of Streptococcus suis in tonsils from live pigs in France. Can J Vet Res 2007; 71:14 - 22; PMID: 17193877
- Luey CK, Chu YW, Cheung TK, Law CC, Chu MY, Cheung DT, Kam KM. Rapid pulsed-field gel electrophoresis protocol for subtyping of Streptococcus suis serotype 2. J Microbiol Methods 2007; 68:648 - 50; http://dx.doi.org/10.1016/j.mimet.2006.10.010; PMID: 17157941
- Wang LL, Ye CY, Xu YM, Cui ZG, Jing HQ, Jin D, Du HM, Zhang SY, Bai XM, Zhao AL, et al. [Development of a protocol on pulsed field gel electrophoresis analysis for Streptococcus suis.]. Zhonghua Liu Xing Bing Xue Za Zhi 2008; 29:473 - 7; PMID: 18956681
- Blume V, Luque I, Vela AI, Borge C, Maldonado A, Domínguez L, Tarradas C, Fernández-Garayzábal JF. Genetic and virulence-phenotype characterization of serotypes 2 and 9 of Streptococcus suis swine isolates. Int Microbiol 2009; 12:161 - 6; PMID: 19784922
- Luque I, Blume V, Borge C, Vela AI, Perea JA, Márquez JM, Fernández-Garayzábal JF, Tarradas C. Genetic analysis of Streptococcus suis isolates recovered from diseased and healthy carrier pigs at different stages of production on a pig farm. Vet J 2010; 186:396 - 8; http://dx.doi.org/10.1016/j.tvjl.2009.09.005; PMID: 19800823
- Chang B, Wada A, Ikebe T, Ohnishi M, Mita K, Endo M, Matsuo H, Asatuma Y, Kuramoto S, Sekiguchi H, et al. Characteristics of Streptococcus suis isolated from patients in Japan. Jpn J Infect Dis 2006; 59:397 - 9; PMID: 17186962
- Vela AI, Goyache J, Tarradas C, Luque I, Mateos A, Moreno MA, Borge C, Perea JA, Domínguez L, Fernández-Garayzábal JF. Analysis of genetic diversity of Streptococcus suis clinical isolates from pigs in Spain by pulsed-field gel electrophoresis. J Clin Microbiol 2003; 41:2498 - 502; http://dx.doi.org/10.1128/JCM.41.6.2498-2502.2003; PMID: 12791872
- Berthelot-Hérault F, Marois C, Gottschalk M, Kobisch M. Genetic diversity of Streptococcus suis strains isolated from pigs and humans as revealed by pulsed-field gel electrophoresis. J Clin Microbiol 2002; 40:615 - 9; http://dx.doi.org/10.1128/JCM.40.2.615-6192002; PMID: 11825980
- Marois C, Le Devendec L, Gottschalk M, Kobisch M. Molecular characterization of Streptococcus suis strains by 16S-23S intergenic spacer polymerase chain reaction and restriction fragment length polymorphism analysis. Can J Vet Res 2006; 70:94 - 104; PMID: 16639941
- Wang HM, Ke CW, Pan WB, Ke BX, Chen JD, Deng XL, Liu MZ, Chen GR, Yang XF, Zhu ZY. [MLST typing of Streptococcus suis isolated from clinical patients in Guangdong Province in 2005]. Nan Fang Yi Ke Da Xue Xue Bao 2008; 28:1438 - 41; PMID: 18753081
- Chen L, Song Y, Wei Z, He H, Zhang A, Jin M. Antimicrobial susceptibility, tetracycline and erythromycin resistance genes, and multilocus sequence typing of Streptococcus suis isolates from diseased pigs in China. J Vet Med Sci 2013; 75:583 7; PMID: 23292102
- King SJ, Leigh JA, Heath PJ, Luque I, Tarradas C, Dowson CG, Whatmore AM. Development of a multilocus sequence typing scheme for the pig pathogen Streptococcus suis: identification of virulent clones and potential capsular serotype exchange. J Clin Microbiol 2002; 40:3671 - 80; http://dx.doi.org/10.1128/JCM.40.10.3671-3680.2002; PMID: 12354864
- Li W, Ye C, Jing H, Cui Z, Bai X, Jin D, Zheng H, Zhao A, Xu Y, Gottschalk M, et al. Streptococcus suis outbreak investigation using multiple-locus variable tandem repeat number analysis. Microbiol Immunol 2010; 54:380 - 8; PMID: 20618684
- Chatellier S, Gottschalk M, Higgins R, Brousseau R, Harel J. Relatedness of Streptococcus suis serotype 2 isolates from different geographic origins as evaluated by molecular fingerprinting and phenotyping. J Clin Microbiol 1999; 37:362 - 6; PMID: 9889219
- Madsen LW, Boye M, Jensen HE. An enzyme-based in situ hybridisation method for the identification of Streptococcus suis.. APMIS 2001; 109:665 - 9; http://dx.doi.org/10.1034/j.1600-0463.2001.d01-130.x; PMID: 11890569
- Boye M, Feenstra AA, Tegtmeier C, Andresen LO, Rasmussen SR, Bille-Hansen V. Detection of Streptococcus suis by in situ hybridization, indirect immunofluorescence, and peroxidase-antiperoxidase assays in formalin-fixed, paraffin-embedded tissue sections from pigs. J Vet Diagn Invest 2000; 12:224 - 32; http://dx.doi.org/10.1177/104063870001200305; PMID: 10826835
- Vecht U, Wisselink HJ, Anakotta J, Smith HE. Discrimination between virulent and nonvirulent Streptococcus suis type 2 strains by enzyme-linked immunosorbent assay. Vet Microbiol 1993; 34:71 - 82; http://dx.doi.org/10.1016/0378-1135(93)90008-U; PMID: 8447081
- del Campo Sepúlveda EM, Altman E, Kobisch M, D’Allaire S, Gottschalk M. Detection of antibodies against Streptococcus suis capsular type 2 using a purified capsular polysaccharide antigen-based indirect ELISA. Vet Microbiol 1996; 52:113 - 25; http://dx.doi.org/10.1016/0378-1135(96)00056-9; PMID: 8914256
- Kataoka Y, Yamashita T, Sunaga S, Imada Y, Ishikawa H, Kishima M, Nakazawa M. An enzyme-linked immunosorbent assay (ELISA) for the detection of antibody against Streptococcus suis type 2 in infected pigs. J Vet Med Sci 1996; 58:369 - 72; http://dx.doi.org/10.1292/jvms.58.369; PMID: 8741273
- Chen K, Han H, Luo Z. Streptococcus suis II immunoassay based on thorny gold nanoparticles and surface enhanced Raman scattering. Analyst 2012; 137:1259 - 64; http://dx.doi.org/10.1039/c2an15997j; PMID: 22282767
- Du H, Huang W, Xie H, Ye C, Jing H, Ren Z, Xu J. The genetically modified suilysin, rSLY(P353L), provides a candidate vaccine that suppresses proinflammatory response and reduces fatality following infection with Streptococcus suis.. Vaccine 2013; 31:4209 - 15; http://dx.doi.org/10.1016/j.vaccine.2013.07.004; PMID: 23856333
- Takamatsu D, Osaki M, Tharavichitkul P, Takai S, Sekizaki T. Allelic variation and prevalence of serum opacity factor among the Streptococcus suis population. J Med Microbiol 2008; 57:488 - 94; http://dx.doi.org/10.1099/jmm.0.47755-0; PMID: 18349370
- Li W, Hu X, Liu L, Chen H, Zhou R. Induction of protective immune response against Streptococcus suis serotype 2 infection by the surface antigen HP0245. FEMS Microbiol Lett 2011; 316:115 - 22; http://dx.doi.org/10.1111/j.1574-6968.2010.02200.x; PMID: 21204934
- de Buhr N, Neumann A, Jerjomiceva N, von Köckritz-Blickwede M, Baums CG. Streptococcus suis DNase SsnA contributes to degradation of neutrophil extracellular traps (NETs) and evasion of NET-mediated antimicrobial activity. Microbiology 2014; 160:385 - 95; http://dx.doi.org/10.1099/mic.0.072199-0; PMID: 24222615
- Segura M, Vadeboncoeur N, Gottschalk M. CD14-dependent and -independent cytokine and chemokine production by human THP-1 monocytes stimulated by Streptococcus suis capsular type 2. Clin Exp Immunol 2002; 127:243 - 54; http://dx.doi.org/10.1046/j.1365-2249.2002.01768.x; PMID: 11876746
- Vanier G, Segura M, Lecours MP, Grenier D, Gottschalk M. Porcine brain microvascular endothelial cell-derived interleukin-8 is first induced and then degraded by Streptococcus suis.. Microb Pathog 2009; 46:135 - 43; http://dx.doi.org/10.1016/j.micpath.2008.11.004; PMID: 19100324
- Lachance C, Segura M, Dominguez-Punaro MC, Wojewodka G, De Sanctis JB, Radzioch D, Gottschalk M. Deregulated balance of omega-6 and omega-3 following infection by the zoonotic pathogen Streptococcus suis.. Infect Immun 2014; (forthcoming; http://dx.doi.org/10.1128/IAI.01524-13; PMID: 24549326
- Huang D, Zhu H, Lin H, Xu J, Lu C. First insights into the protective effects of a recombinant swinepox virus expressing truncated MRP of Streptococcus suis type 2 in mice. Berl Munch Tierarztl Wochenschr 2012; 125:144 - 52; PMID: 22515033
- Tan C, Liu M, Liu J, Yuan F, Fu S, Liu Y, Jin M, Bei W, Chen H. Vaccination with Streptococcus suis serotype 2 recombinant 6PGD protein provides protection against S. suis infection in swine. FEMS Microbiol Lett 2009; 296:78 - 83; http://dx.doi.org/10.1111/j.1574-6968.2009.01617.x; PMID: 19459970
- Li W, Hu X, Liu L, Chen H, Zhou R. Induction of protective immune response against Streptococcus suis serotype 2 infection by the surface antigen HP0245. FEMS Microbiol Lett 2011; 316:115 - 22; http://dx.doi.org/10.1111/j.1574-6968.2010.02200.x; PMID: 21204934
- Zhang YM, Shao ZQ, Wang J, Wang L, Li X, Wang C, Tang J, Pan X. Prevalent distribution and conservation of Streptococcus suis Lmb protein and its protective capacity against the Chinese highly virulent strain infection. Microbiol Res 2013; forthcoming http://dx.doi.org/10.1016/j.micres.2013.09.007; PMID: 24120016
