Figures & data
Table 1. Experiment initial conditions.
Table 2. Similar experimental data in JSR.
Figure 1. The oxidation of methane in a JSR at atmospheric pressure and φ = 0.3 and 1. Upper panel compares experimental (symbols) and simulation (blue line shows the steady-state solution, and the filled pattern shows oscillation range and variation). The lower panel shows a dynamic simulation of the cases at the starting point of oscillation. Data of T3 is from Ref. (El Bakali et al., Citation2004); T4 and T5 are from Ref. (Le Cong et al., Citation2008).
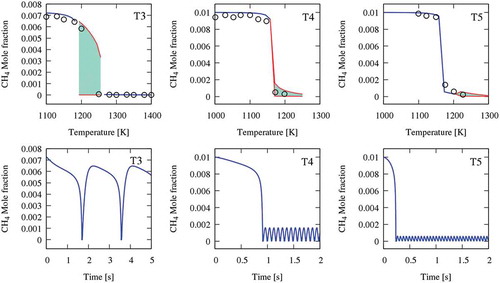
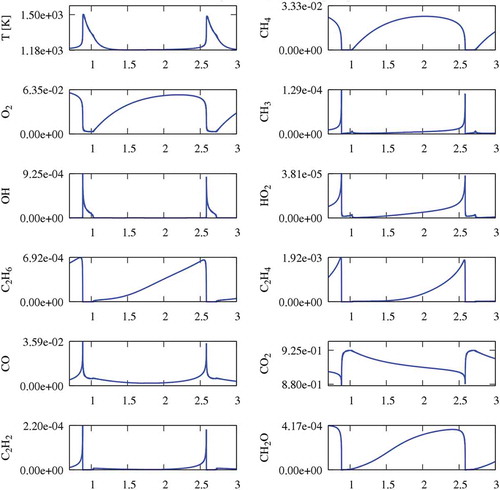
Figure 2. Methane oxidation in a JSR at P = 1.1 atm, τ = 0.5 s, and φ = 1 diluted with 90% of N2. Oscillation ranges are highlighted with the filled pattern.
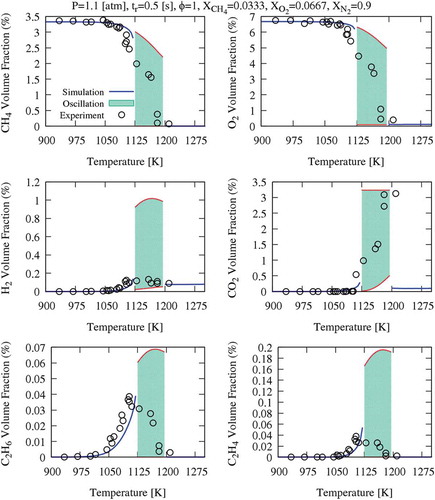
Figure 3. Range of temperature oscillations (ΔT = T-Tin) and CH4 conversion versus inlet temperature, for the different dilution systems.
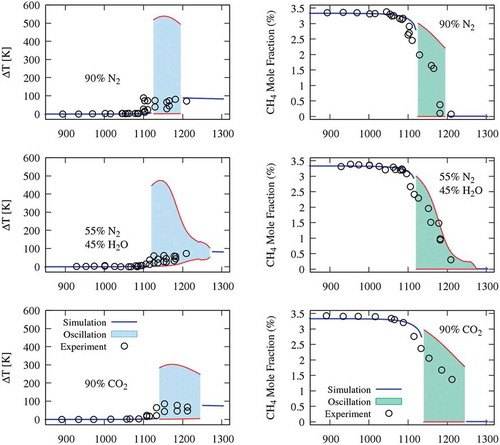
Figure 4. Temperature and CH4 profiles at 1185 K, in CO2 diluted system. Temperature and methane profile close to the peak temperature (right panel).
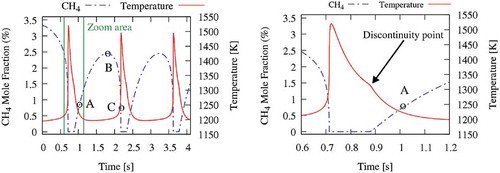
Figure 5. Sensitivity analysis of methane concentration in the CO2 diluted system and Tin = 1185 K (points A, B and C are shown in ).
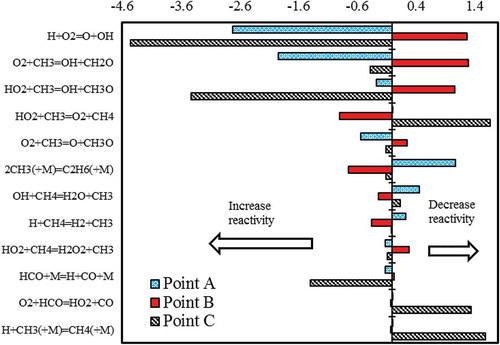
Figure 8. Oscillations of temperature and CH4 concentration in CO2 diluted systems. Tin = 1140 K (black line); Tin = 1185 K (dotted-dashed line); Tin = 1245 K (red line).
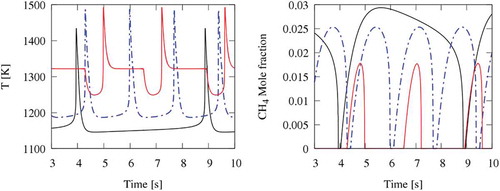
Figure 9. Oscillations of temperature and CH4 concentration in N2, CO2, and (N2+H2O) diluted systems. CO2 (black lines); N2 (dotted-dashed lines); N2+H2O (red lines).
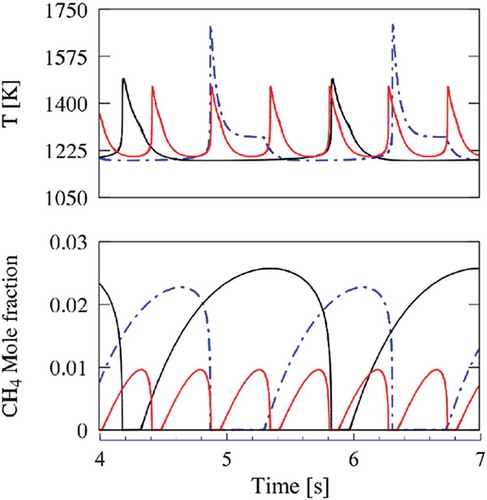
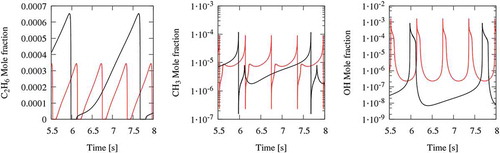
Figure 10. Comparison between cyclic oscillations in N2+H2O (red lines) and CO2 diluted systems (black lines). C2H6, CH3, and OH concentration profiles.