Figures & data
Figure 1. Potential–pH diagram for Se based on the thermodynamic data reviewed and compiled by Olin et al. [Citation2]. The total concentration of Se is 9.3×10−4 mol kg−1. The line with arrow points indicates the scan region in this study (0.449↔1.439 V vs. SHE and pH = 1.13 ± 0.26).
![Figure 1. Potential–pH diagram for Se based on the thermodynamic data reviewed and compiled by Olin et al. [Citation2]. The total concentration of Se is 9.3×10−4 mol kg−1. The line with arrow points indicates the scan region in this study (0.449↔1.439 V vs. SHE and pH = 1.13 ± 0.26).](/cms/asset/4fa517e1-b253-4b6d-adc5-258b622cd84c/tnst_a_872059_f0001_oc.jpg)
Figure 3. Dependence of the anodic peak current on v1/2. The eight filled circles corresponding to each scan rate in the figure are obtained from the 40th cyclic voltammogram after repetitive potential cycling measured in a solution containing H2SeO3 . The solid line is the linear least-squares fit of data. The linear dependence of the anodic peak current on v1/2 is indicative of the diffusion-controlled process.
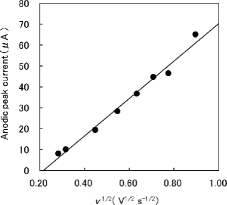
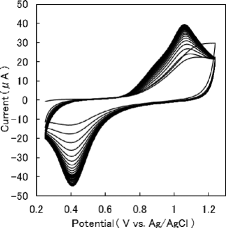
Table 1. Ep(Ox), Ep(Red) and E1/2(T, Im) of the 40th cyclic voltammogram after repetitive potential cycling, measured at different scan rates in a solution containing H2SeO3 .
Table 2. Summary of the cyclic voltammetry experiments to measure the half-wave potentials for the HSeO4−/H2SeO3 couple in acidic sodium nitrate solutions as a function of mNa+.
Table 3. Values of Yintercept, ϵT(HSeO4−, Na+), EAg/AgCl(T, 0), log10 K1(T, 0) and . The intercept and slope of each line in correspond to Y intercept and ϵT(HSeO4−, Na+), respectively, at each temperature.
Figure 4. Determination of the standard redox potential for the HSeO4−/H2SeO3 couple using SIT. Experimental data as a function of are shown as the solid bars with a vertical bar showing the standard deviation in each data point. The solid line is a weighted linear regression [Citation24] of data. The slope and intercept of each line correspond to ϵT(HSeO4−, Na+) and Yintercept, respectively, at each temperature.
, where J = RT/2F log10 e. Yintercept = [
– EAg/AgCl(T, 0)]/J + log10 K1(T, 0).
![Figure 4. Determination of the standard redox potential for the HSeO4−/H2SeO3 couple using SIT. Experimental data as a function of are shown as the solid bars with a vertical bar showing the standard deviation in each data point. The solid line is a weighted linear regression [Citation24] of data. The slope and intercept of each line correspond to ϵT(HSeO4−, Na+) and Yintercept, respectively, at each temperature. , where J = RT/2F log10 e. Yintercept = [ – EAg/AgCl(T, 0)]/J + log10 K1(T, 0).](/cms/asset/04d0bb24-e61c-4314-99d0-e520c2f4fbdb/tnst_a_872059_f0004_b.gif)
Figure 5. Determination of the molar entropy for the HSeO4−/H2SeO3 couple. The HSeO4−/H2SeO3 standard redox potential as a function of temperature is shown as the filled circle with vertical bar showing the standard deviation in each data point. The solid line is a weighted linear regression [Citation24] of data. The slope of this line corresponds toΔrS0m/2F = −0.3 ± 0.1 mV K− 1.
![Figure 5. Determination of the molar entropy for the HSeO4−/H2SeO3 couple. The HSeO4−/H2SeO3 standard redox potential as a function of temperature is shown as the filled circle with vertical bar showing the standard deviation in each data point. The solid line is a weighted linear regression [Citation24] of data. The slope of this line corresponds toΔrS0m/2F = −0.3 ± 0.1 mV K− 1.](/cms/asset/45112fff-cfdd-45f3-a693-e419364ad7a7/tnst_a_872059_f0005_b.gif)
Table 4. Standard thermodynamic data for the Se(VI)/(IV) couple.
Figure 6. Dependence of the ion interaction coefficient ϵT(HSeO4−, Na+) on temperature. The ion interaction coefficient ϵT(HSeO4−, Na+) as a function of temperature is shown as the filled circle with vertical bar showing the standard deviation in each data point. The solid line is a weighted linear regression [Citation24] of data. The slope of this line corresponds to ∂ϵ/∂T = −0.002 ± 0.002 kg mol−1 K−1.
![Figure 6. Dependence of the ion interaction coefficient ϵT(HSeO4−, Na+) on temperature. The ion interaction coefficient ϵT(HSeO4−, Na+) as a function of temperature is shown as the filled circle with vertical bar showing the standard deviation in each data point. The solid line is a weighted linear regression [Citation24] of data. The slope of this line corresponds to ∂ϵ/∂T = −0.002 ± 0.002 kg mol−1 K−1.](/cms/asset/d8f91ca6-0689-4f84-9b7e-206fde082fdc/tnst_a_872059_f0006_b.gif)