Figures & data
Fig. 1 Topographic map of the Skippers Creek catchment. Bullendale fault zone and other major fault zones are drawn after Begbie & Craw (Citation2006), Craw et al. (Citation2006), and MacKenzie et al. (Citation2007) and alteration halos (hatched) are added after Craw et al. (Citation2006). Major rock types are also shown (grey shades). Rivers and sites discussed in the text are annotated and water samples positions and their labels are added. The inset digital elevation model of the South Island of New Zealand shows the extent of the Otago Schist and the location of Skippers Creek catchment.
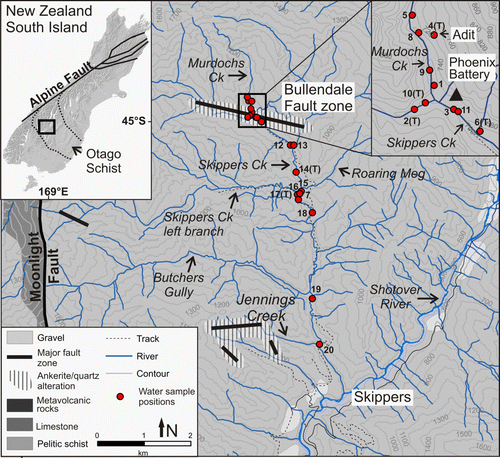
Fig. 2 Schematic site outline of the Phoenix battery site showing the principal water flow directions and sampling positions for water. Locations of mine waste sampling Profiles A, B, and C are indicated.
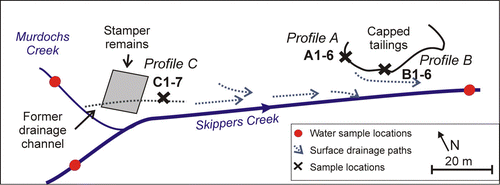
Fig. 3 (A) Sampling details in Profiles A, B and C (). Italic sample labels indicate that the material was not necessarily in situ. (B) Analytical results (based on XRF), including As, Fe and S concentrations, and mineralogy (based on XRD and EDS methods). Minerals are classified as present in major (M), subordinate (S) and trace (T) amounts. The degree of cementation is indicated with strongly cemented ‘SC’, weakly cemented ‘WC’, and friable ‘F’. The abbreviation ‘b.d.’ indicates that the analyte was below the detection limit.
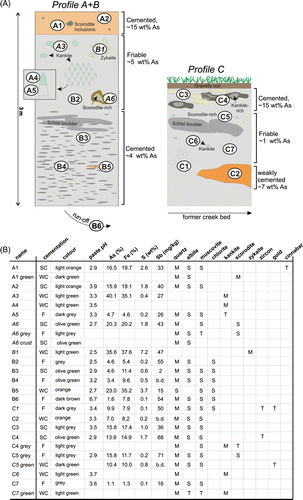
Fig. 4 Ternary diagram based on wt% of As-Fe-S. Samples of discrete mineral phases are indicated as hollow symbols. The composition of kankite/scorodite, zykaite, arsenopyrite, and pyrite are added for comparison (full symbols).
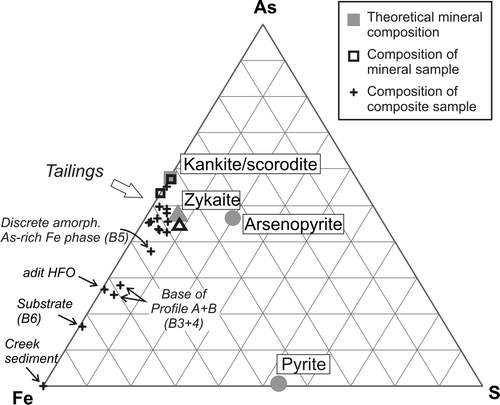
Fig. 5 Arsenic phases and their morphology. (A) Photograph of discrete centimetre-scale globular aggregations of zykaite (sample B1) and kankite (sample A3). (B) Backscatter electron (BSE) image of a kankite aggregate with an inset of a microscopic photograph of kankite (sample A3). (C) Reflected light microscopic image (plane polarised light) of a cemented portion of sample A6. Upper left half is cemented with pale green iron arsenate (As-Fe), and lower right is cemented with brown ferrihydrite (Fe) containing minor As. Boundary between two cement types is dashed. (D) BSE image of interstitial globular growth of As-rich Fe phases (sample C1). (E) BSE image of As-rich ferrihydrite coatings on quartz with an inset of microscopic photograph showing the typical orange colour of this As-bearing phase (sample C2). (F) BSE image of an arsenopyrite grain pseudomorphically replaced by iron arsenate (sample C1).
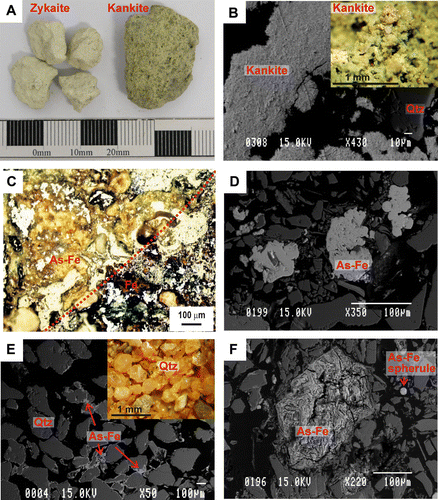
Fig. 6 Piper diagram of surface water collected in Skippers Creek catchment upstream and downstream of the Phoenix battery ( and ).
Table 1 Major ion data including dissolved and sediment concentrations of As and Sb
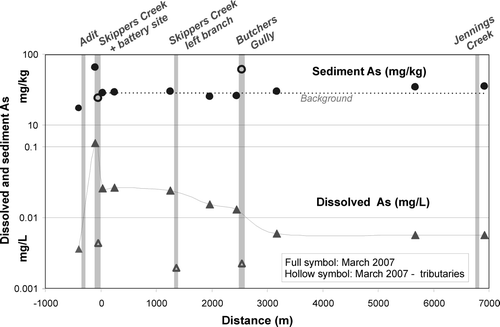
Fig. 7 Major ion, dissolved As and sulfate concentrations, and As flux with distance from the Phoenix battery.
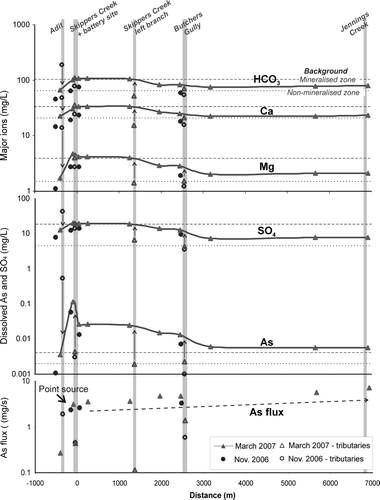
Fig. 9 (A) Eh-pH diagram with stability fields of As species, including scorodite and scorodite-like material, arsenopyrite, and dissolved species (Craw et al. Citation2003). The environmental conditions of the tailings originally and at present are indicated based on observations. (B) Solubility of scorodite after Krause & Ettel (Citation1988). Without external modification (‘closed system’) the pH of the mine residues remain acidic and iron arsenate solubility is low. If neutralisation of mine residues occurs from external sources (‘open system’) iron arsenate solubility increases by several orders of magnitude.
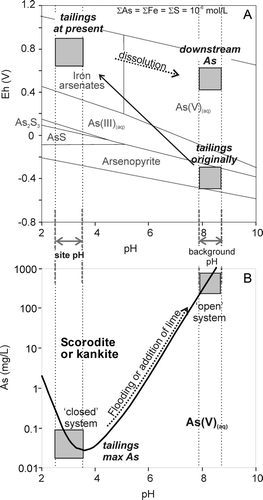
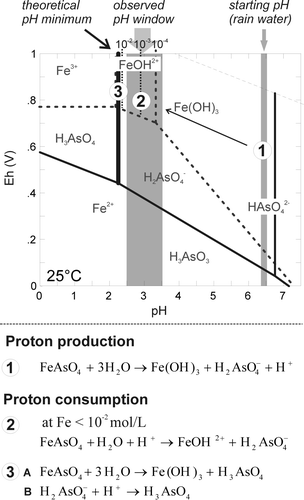
Fig. 10 Eh-pH diagram of dissolved As species (solid lines) and Fe species (broken line) at 10−4 mol/L. The observed pH window of mine residues is added. Reactions controlling pH are listed and the environment they occur in is indicated by circled numbers in the Eh-pH diagram.