Figures & data
Figure 1. Vegetative growth and physiological data showing dry weight after a 7-day acclimation period (A) and dry weight (B) and leaf chlorophyll concentration shown as SPAD units (C) after 14 days of exposure to main saline-alkaline stress (SAS) treatment (B). plants were pre-treated with various concentrations of NaCl (S) (2.5, 5.0, and 10 mM), H2O2 (OX) (1.0, 5.0, and 10.0 µM), and NaHCO3 (SA) (0.5, 1.0, and 2.5 mM) for 7 days and subjected to 50 mM Na, pH 8.25 for 14 days. the data represents means ± standard errors from five biological replicates. different letters indicate significant differences by tukey’s multiple comparison tests at P < 0.05.
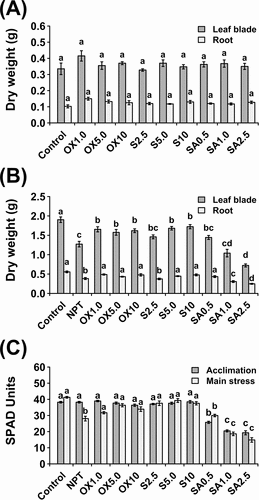
Figure 2. Rhizosphere acidification data showing daily pH changes in the growth medium during a 14-day period of main saline-alkaline stress (SAS) treatment in plants pre-treated with various concentrations of NaCl (A), H2O2 (B), and NaHCO3 (C) versus non-pre-treated seedlings. plants were pre-treated with various concentrations of NaCl (S) (2.5, 5.0, and 10 mM), H2O2 (OX) (1.0, 5.0, and 10.0 µM), and NaHCO3 (SA) (0.5, 1.0, and 2.5 mM) for 7 days and subjected to 50 mM Na, pH 8.25 for 14 days.
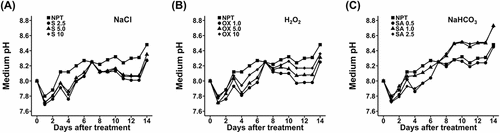
Figure 3. Macro and micronutrient analyses showing concentrations of potassium (A), sodium (B), phosphorus (C), iron (D), boron (E), and zinc (F) in leaf blades and roots after 14 days of exposure to main saline-alkaline stress (SAS) treatment. plants were pre-treated with 0 (NPT), 10 mM NaCl (S10), 10 µM H2O2 (OX10), and 0.5 mM NaHCO3 (SA0.5) for 7 days and subjected to 50 mM Na, pH 8.25 for 14 days. the data represents means ± standard errors from four biological replicates. different letters indicate significant differences by tukey’s multiple comparison tests at P < 0.05.
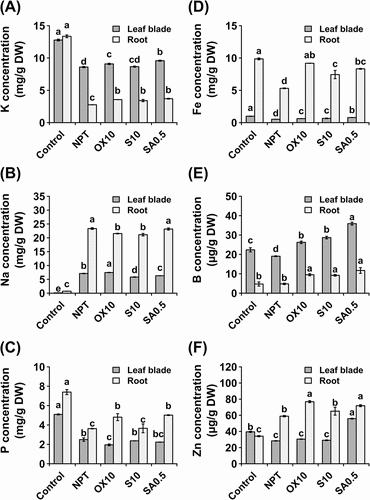
Figure 4. Physiological experiments for studying K+ and Na+ homeostasis. K+ leakage in roots of 7-day pre-treated (PT) and non-pre-treated (NPT) seedlings subjected to 50 mM Na, pH 8.50 for 24 h (A). Efflux of Na+ from roots of 7-day PT and NPT seedlings, subjected to 50 mM Na, pH 8.50 for 24 h and deionized water for 2 h (B). Concentrations of Na+ (C) and chlorophyll (D) in excised leaves from 7-day PT and NPT plants subjected to 30 mM Na, pH 8.0 for 3 days. Plants were pre-treated with 0 (NPT), 10 mM NaCl (S10), 10 µM H2O2 (OX10), and 0.5 mM NaHCO3 (SA0.5) for 7 days and subjected to the above experiments. the data represents means ± standard errors from four biological replicates. different letters indicate significant differences by tukey’s multiple comparison tests at P < 0.05.
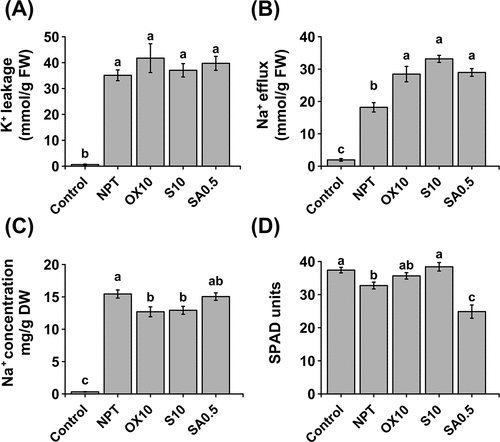
Figure 5. Oxidative stress, membrane integrity, and cell viability parameters showing leakage of electrolytes (A), malondialdehyde concentrations (B), Evans Blue staining (C), and concentrations of hydrogen peroxide (D) in leaf blades and roots after 14 days of main saline-alkaline stress (SAS) treatment. Plants were pre-treated with 0 (NPT), 10 mM NaCl (S10), 10 µM H2O2 (OX10), and 0.5 mM NaHCO3 (SA0.5) for 7 days and subjected to 50 mM Na, pH 8.25 for 14 days. The data represents means ± standard errors from four biological replicates. different letters indicate significant differences by Tukey’s multiple comparison tests at P < 0.05.
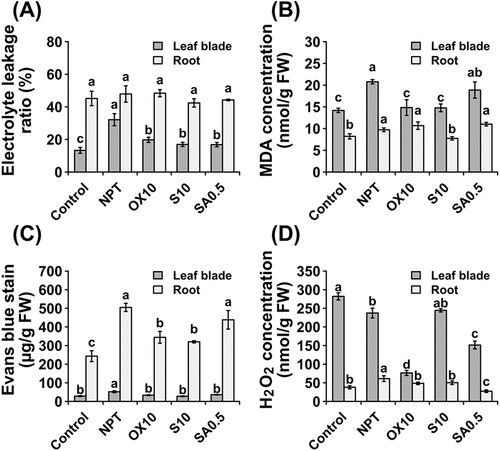
Figure 6. Antioxidative enzyme activities showing activities of catalase (A), guaiacol peroxidase (B), ascorbate peroxidase (C), glutathione reductase (D), and superoxide dismutase (E) and protein concentration (F) in leaf blades and roots after 14 days of exposure to main saline-alkaline stress (SAS) treatment. Plants were pre-treated with 0 (NPT), 10 mM NaCl (S10), 10 µM H2O2 (OX10), and 0.5 mM NaHCO3 (SA0.5) for 7 days and subjected to 50 mM Na, pH 8.25 for 14 days. The data represents means ± standard errors from four biological replicates. Different letters indicate significant differences by Tukey’s multiple comparison tests at P < 0.05.
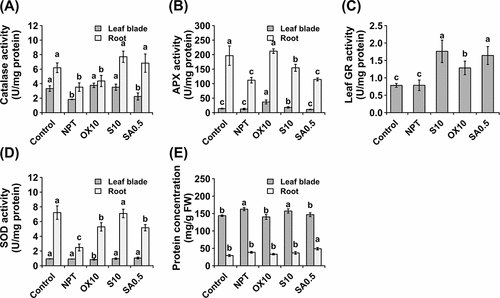
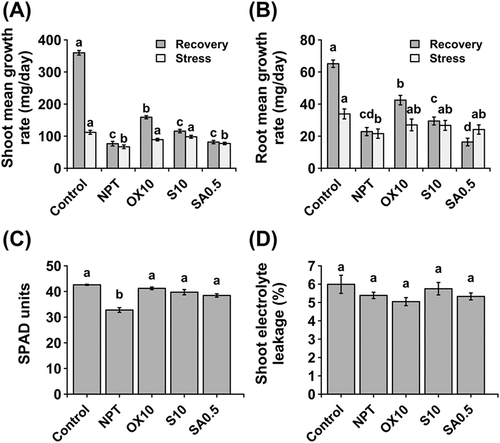
Figure 7. Effect of pre-treatments on recovery of plants after exposure to saline-alkaline stress. Comparative mean growth rates during and after stress in shoots (A) and roots (B). Chlorophyll concentration (C) and membrane integrity assessed by the electrolyte leakage method in shoots (D) at the end of the 10-day recovery period. ‘Stress’ group represents mean growth rate of plants during the 14 day growth period under main saline-alkaline stress (SAS) (50 mM Na+, pH 8.25) after pre-treatment with 0 (NPT), 10 mM NaCl (S10), 10 µM H2O2 (OX10), and 0.5 mM NaHCO3 (SA0.5) for 7 days. ‘Recovery’ group represents the growth rate of the same plants upon completion of main saline-alkaline stress (SAS) and subjecting them to recovery under normal conditions (0 mM Na+, pH 5.5) for 10 days. The data represents means ± standard errors from five biological replicates. different letters indicate significant differences by tukey’s multiple comparison tests at P < 0.05.