Figures & data
Figure 1. Poisson example in Section 2.4. (a), (c) expected utility U(x) against x, with Monte Carlo evaluations at the coordinate-design points and GP emulator
; (b), (d) median probability p†I of accepting the candidate point against the current point, xC. [Coordinate-designs are: ξ1 for (a), (b); ξ2 for (c), (d)].
![Figure 1. Poisson example in Section 2.4. (a), (c) expected utility U(x) against x, with Monte Carlo evaluations U˜(x) at the coordinate-design points and GP emulator U^(x); (b), (d) median probability p†I of accepting the candidate point against the current point, xC. [Coordinate-designs are: ξ1 for (a), (b); ξ2 for (c), (d)].](/cms/asset/c8e39f62-1517-4820-8ba3-e5088fa2014f/utch_a_1251495_f0001_b.gif)
Figure 2. (a), (b) Trace plots of for each iteration of the ACE algorithm for SIG and pseudo-Bayesian D-optimality utilities, respectively; in each plot, the black line shows the trace of the expected utility for the best design; (c) designs found from the ACE algorithm: unrestricted SIG-optimal, pseudo-Bayesian D-optimal, Beta DRS SIG-optimal, together with the Ryan et al. (Citation2014) Beta DRS SIG-optimal designs; (d) boxplots for 20 evaluations of
for designs from these four methodologies; (e) approximate expected utility surface for SIG as a function of the Beta DRS parameters; parameter values corresponding to the Ryan et al. (Citation2014) and the ACE DRS designs are marked.
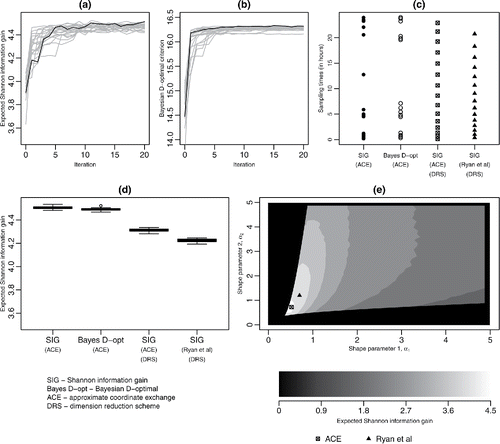
Figure 3. Results from 20 evaluations of (a) for SIG-optimal, pseudo-Bayesian D-optimal, and maximin Latin hypercube designs, and (b)
for NSEL-optimal, pseudo-Bayesian A-optimal, and maxmin Latin hypercube designs, for homogenous logistic regression; (c) and (d) show the same evaluations for hierarchical logistic regression. For the latter two plots, for each value of n, 20 different random assignments are made of the points of the Latin hypercube design to the G groups, and each resulting design is evaluated 20 times. For each design, the central plotting symbol denotes the mean expected Shannon information gain or expected average posterior variance, with the two horizontal lines denoting the minimum and maximum of these quantities.
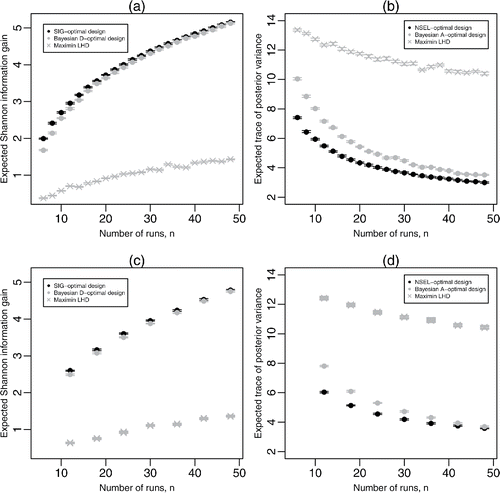
Table 1. Approximate posterior model probabilities, π(u | y), for the beetle mortality data.
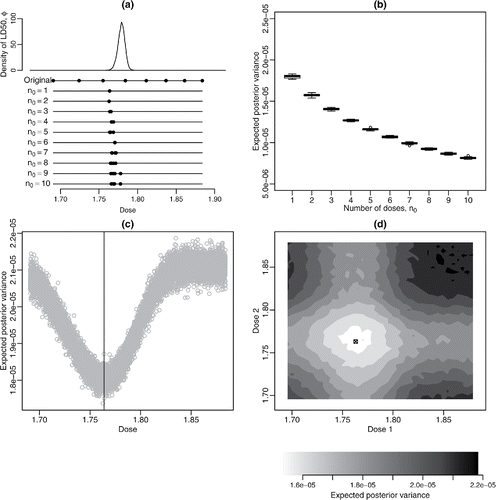
Figure 4. (a) Posterior density for LD50, the original experimental doses and optimal doses (in mg/L) for each value of n0; (b) boxplots of 20 evaluations of for each n0 for the NSEL-optimal designs; (c) negative approximate expected utility
against dose for n0 = 1; the vertical line indicates δ⋆. (d) negative approximate expected utility
against dose for n0 = 2; ⊠ indicates δ⋆.