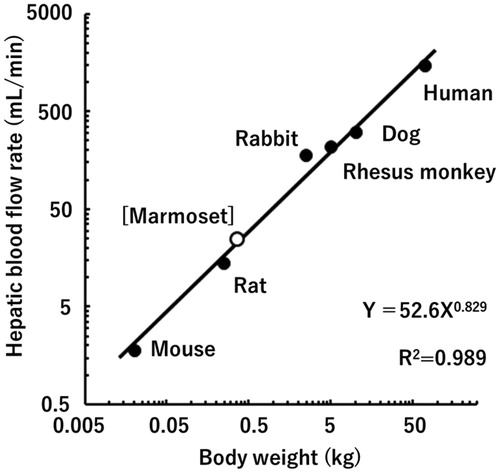
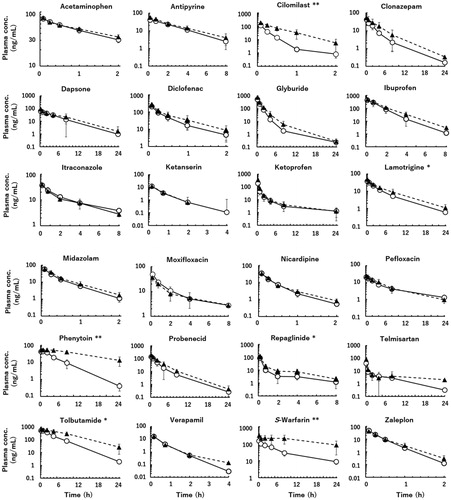
Figures & data
Table 1. Physicochemical properties, cassette-dose cocktail group numbers, LC–MS/MS conditions, and reported enzymes responsible for metabolism in humans of the 24 model compounds tested in this study.
Table 2. Phenotypes and genotypes of polymorphic CYP2C19 for the six marmosets used in this study and their frequencies in a breeder cohort.
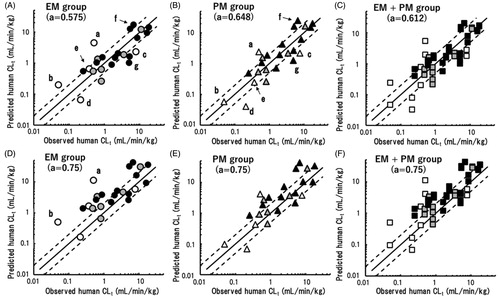
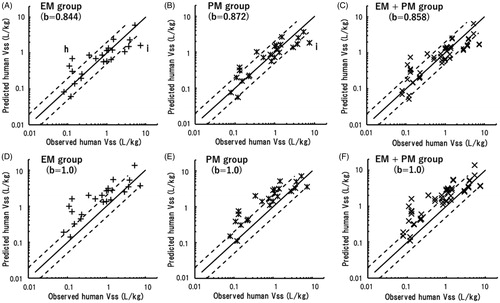
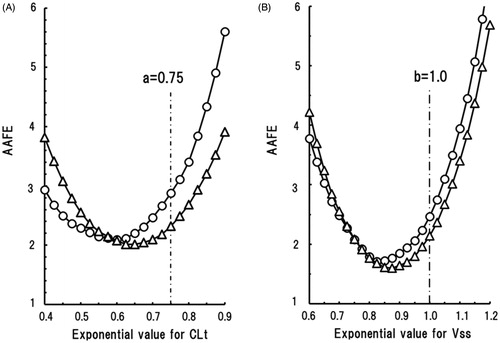
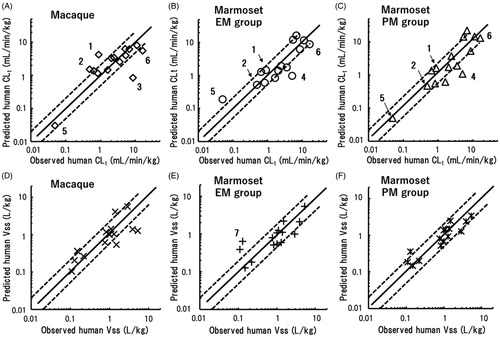
Table 3. Total clearance (CLt) values and distribution volumes at steady state (Vss) observed in marmosets and predicted for humans by single-species allometric scaling based on pharmacokinetic data sets in marmosets.
Table 4. Average absolute fold errors (AAFEs) and the percentages of CLt and Vss values within two- and threefold errors compared with the observed values in humans for values predicted by single-species allometric scaling in extensive (EM) and poor (PM) metaboliser marmosets.
Table 5. Comparison of human total clearance (CLt) values and volumes of distribution (Vss) predicted using single-species allometric scaling from reported data in macaques and observed data in marmosets for 15 of the 24 test compounds.
Table 6. Comparison of predictabilities of human CLt and Vss values for 15 compounds using single-species allometric scaling based on data from macaques and marmosets.