Figures & data
Figure 1. Reductive amination of cyclohexanone with benzylamine. Adapted fromRef. [Citation10] Open access.
![Figure 1. Reductive amination of cyclohexanone with benzylamine. Adapted fromRef. [Citation10] Open access.](/cms/asset/c28ef87e-2779-4100-9e28-c6e3224c2c80/lctr_a_1942689_f0001_b.gif)
Figure 2. Reductive one-pot amination of nitrobenzene with benzaldehyde adapted from.[Citation28–30] Copyright permission from John Wiley & Sons and from Royal Society of Chemistry.
![Figure 2. Reductive one-pot amination of nitrobenzene with benzaldehyde adapted from.[Citation28–30] Copyright permission from John Wiley & Sons and from Royal Society of Chemistry.](/cms/asset/a6e3466c-e6aa-4d10-9805-152c3649590c/lctr_a_1942689_f0002_b.gif)
Figure 3. A simplified scheme for reductive amination of an aldehyde/ketone with a primary amine and one-pot reductive amination of nitroarene to the corresponding secondary amine using an aldehyde/ketone. Aldehyde can be prepared from an alcohol.
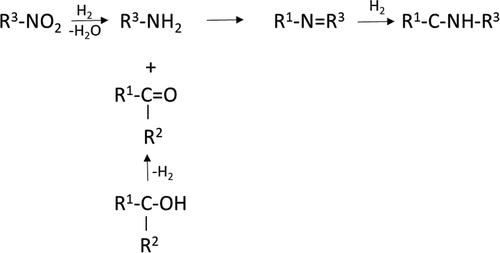
Table 1. Amination of aldehyde and ketones with amines using molecular hydrogen.a Notation: AM amine, IM imine, AL alcohol, KET ketone, ALD aldehyde.
Table 2. Reductive amination of α-methyltryptamine derivative to N-alkyl-α-methyltryptamine derivative after 18 h. Adapted from Ref. [Citation27] Copyright permission from Elsevier Ltd.
Figure 4. Reductive amination of α-methyltryptamine derivative into N,N-dialkyl-α-methyltryptamine derivative adapted from.[Citation27] Copyright permission from Elsevier Ltd.
![Figure 4. Reductive amination of α-methyltryptamine derivative into N,N-dialkyl-α-methyltryptamine derivative adapted from.[Citation27] Copyright permission from Elsevier Ltd.](/cms/asset/85cf496b-d5e9-4240-83df-2882b2ba022b/lctr_a_1942689_f0004_b.gif)
Table 3. Solvent effect in reductive amination of furfural with aniline over Pt/SiO2–SO3 catalyst. Reaction conditions: furfural, 6 mmol; aniline, 5 mmol; catalyst, 0.15 mol%; ethyl acetate, 40 mL; H2 pressure, 5 MPa; room temperature; 8 h, adapted from Ref .[Citation26] Copyright from Elsevier Ltd.
Table 4. Reductive amination of AMF with primary amines R-NH2 over Pt/Al2O3 catalyst adapted from Ref. [Citation24].
Figure 5. Product distributions of reductive amination of cyclohexanone over Group VIII metal-based catalysts in methanol and water. Reaction conditions: Cyclohexanone 1 mmol, catalyst 0.02 g, solvent 3 mL, under 9 bar hydrogen, T = 25°C, agitator speed = 1000 rpm, time = 2 h adapted from Ref. [Citation25] Notation: 1 methanol, 2 water as a solvent. Copyright from Elsevier Ltd.
![Figure 5. Product distributions of reductive amination of cyclohexanone over Group VIII metal-based catalysts in methanol and water. Reaction conditions: Cyclohexanone 1 mmol, catalyst 0.02 g, solvent 3 mL, under 9 bar hydrogen, T = 25°C, agitator speed = 1000 rpm, time = 2 h adapted from Ref. [Citation25] Notation: 1 methanol, 2 water as a solvent. Copyright from Elsevier Ltd.](/cms/asset/533c5865-3b1a-4236-a8dd-609f859f2055/lctr_a_1942689_f0005_b.gif)
Figure 6. Concentration profiles in HMF amination with aniline over Pd supported on poly-para-phenylenediamine modified metal organic framework catalyst UiO-67 in ethanol at 50°C under 5 bar hydrogen adapted from.[Citation68] Notation: (■) HMF conversion, (▲) imine and (●) amine yield. Copyright permission from Royal Society of Chemistry.
![Figure 6. Concentration profiles in HMF amination with aniline over Pd supported on poly-para-phenylenediamine modified metal organic framework catalyst UiO-67 in ethanol at 50°C under 5 bar hydrogen adapted from.[Citation68] Notation: (■) HMF conversion, (▲) imine and (●) amine yield. Copyright permission from Royal Society of Chemistry.](/cms/asset/638f7338-98a9-4ae4-999e-720bef655747/lctr_a_1942689_f0006_b.gif)
Figure 7. Scheme of reductive amination of AMF with different aromatic and aliphatic amines adapted from.[Citation24] Copyright permission from Elsevier Ltd.
![Figure 7. Scheme of reductive amination of AMF with different aromatic and aliphatic amines adapted from.[Citation24] Copyright permission from Elsevier Ltd.](/cms/asset/a5053073-7135-4eb8-a125-14ed28b241a1/lctr_a_1942689_f0007_b.gif)
Figure 8. Reaction mechanism for formation of an imine from a carbonyl compound and an amine.[Citation82]
![Figure 8. Reaction mechanism for formation of an imine from a carbonyl compound and an amine.[Citation82]](/cms/asset/1de4c64c-bdf4-48b4-b51e-fb75f1d6fb66/lctr_a_1942689_f0008_b.gif)
Table 5. Secondary amines from aldehyde/ketones and amines using other hydrogen sources than molecular hydrogen. Notation: FA formic acid, SILP supported ionic liquid phase. Notation: Y yield, AM amine, S selectivity
Figure 9. Conversion of HMF (■) and concentration profiles of alkyliminefuran (▲), alkylaminofuran (●) and 2,5-bis-(hydroxymethyl)furan (o) in amination of HMF with aniline over Au/TiO2 at 60°C under 20 bar in 1:1 methanol water mixture as a solvent adapted from Ref. [Citation84]. Copyright permission from Royal Society of Chemistry.
![Figure 9. Conversion of HMF (■) and concentration profiles of alkyliminefuran (▲), alkylaminofuran (●) and 2,5-bis-(hydroxymethyl)furan (o) in amination of HMF with aniline over Au/TiO2 at 60°C under 20 bar in 1:1 methanol water mixture as a solvent adapted from Ref. [Citation84]. Copyright permission from Royal Society of Chemistry.](/cms/asset/8105b20b-da34-48e9-aa95-ec57bf79ca1a/lctr_a_1942689_f0009_b.gif)
Figure 10. Pd/C-catalyzed reductive amination of aldehydes with Fe in CO2/H2O system adapted from Ref. [Citation2] Copyright of Elsevier Ltd.
![Figure 10. Pd/C-catalyzed reductive amination of aldehydes with Fe in CO2/H2O system adapted from Ref. [Citation2] Copyright of Elsevier Ltd.](/cms/asset/51ea3dfc-d282-4b5c-9622-ae30ba705615/lctr_a_1942689_f0010_b.gif)
Figure 11. Isotope labeling experiments adapted from Ref. [Citation2] Copyright permission from Elsevier Ltd.
![Figure 11. Isotope labeling experiments adapted from Ref. [Citation2] Copyright permission from Elsevier Ltd.](/cms/asset/e468ea8a-1626-4512-acd2-a08690f2ad65/lctr_a_1942689_f0011_b.gif)
Table 6. Comparison of different methods of N-benzylcyclohexylamine preparation. Notation: Y yield, AM amine, IM imine.
Table 7. The highest yields of secondary amines resulted from amination of aldehydes and ketones with nitrocompounds over heterogeneous catalysts using molecular hydrogen. Notation: nN:nAld and nN:nAld denote the molar ratio of nitro compound to aldehyde and ketone, respectively, cAld and cket denote initial concentrations of aldehyde and ketone, respectively.
Figure 12. Reductive amination of 4-methylfurfural with nitrocyclohexane over Au/TiO2 adapted from Ref. [Citation94] Copyright permission from Elsevier Ltd.
![Figure 12. Reductive amination of 4-methylfurfural with nitrocyclohexane over Au/TiO2 adapted from Ref. [Citation94] Copyright permission from Elsevier Ltd.](/cms/asset/a618b777-013d-4c90-9631-14132498c46a/lctr_a_1942689_f0012_b.gif)
Figure 13. Effect of temperature in reductive amination of benzaldehyde with nitrobenzene over Co supported on mesoporous nitrogen doped carbon (square) adapted from Ref. [Citation62] and over Fe2O3 supported on nitrogen doped graphitic carbon (circle) adapted from Ref. [Citation28] Notation: open symbol imine, solid symbol amine. Copyright permissions from John Wiley & Sons and from Elsevier Ltd.
![Figure 13. Effect of temperature in reductive amination of benzaldehyde with nitrobenzene over Co supported on mesoporous nitrogen doped carbon (square) adapted from Ref. [Citation62] and over Fe2O3 supported on nitrogen doped graphitic carbon (circle) adapted from Ref. [Citation28] Notation: open symbol imine, solid symbol amine. Copyright permissions from John Wiley & Sons and from Elsevier Ltd.](/cms/asset/e14a402e-8299-45bc-96b7-9383f0927953/lctr_a_1942689_f0013_b.gif)
Figure 14. Mechanism for one-pot reductive amination of nitroarenes with aldehydes.[Citation82].
![Figure 14. Mechanism for one-pot reductive amination of nitroarenes with aldehydes.[Citation82].](/cms/asset/862c8d4c-4533-44ad-9d53-50f3d413e3a4/lctr_a_1942689_f0014_oc.jpg)
Figure 15. Effect of temperature in reductive amination of benzaldehyde with nitrobenzene over Co supported on nitrogen doped carbon (CN-600, pyrolyzed at 600°C) (ball) adapted from Ref. [Citation53] and over Co supported on nitrogen doped carbon (CN-800, pyrolyzed at 800°C) (rectangular) adapted from Ref. [Citation56] in the presence of formic acid. Notation: open symbol: imine, solid symbol: amine. Copyright permissions from Elsevier Ltd and from American Chemical Society.
![Figure 15. Effect of temperature in reductive amination of benzaldehyde with nitrobenzene over Co supported on nitrogen doped carbon (CN-600, pyrolyzed at 600°C) (ball) adapted from Ref. [Citation53] and over Co supported on nitrogen doped carbon (CN-800, pyrolyzed at 800°C) (rectangular) adapted from Ref. [Citation56] in the presence of formic acid. Notation: open symbol: imine, solid symbol: amine. Copyright permissions from Elsevier Ltd and from American Chemical Society.](/cms/asset/387ab094-2e2c-4e40-8546-f8d89caf4e71/lctr_a_1942689_f0015_b.gif)
Figure 16. The reaction mechanism for one-pot reductive amination of nitrobenzene with benzaldehyde using the CO/H2O assisted system.[Citation95] Copyright Elsevier Ltd.
![Figure 16. The reaction mechanism for one-pot reductive amination of nitrobenzene with benzaldehyde using the CO/H2O assisted system.[Citation95] Copyright Elsevier Ltd.](/cms/asset/32342116-e21a-41da-bf7d-b169fc51281d/lctr_a_1942689_f0016_oc.jpg)
Table 8. Hydrogen sources other than molecular hydrogen used in amination of benzaldehyde with nitrobenzene over heterogeneous catalysts. Notation: N nitro compound, BA benzaldehyde.
Table 9. One-pot reductive amination of aldehydes with nitroarenes over Ni/H-mZSM-5 with NaBH4 as a reducing agent a.[Citation50].
Figure 17. Concentration profiles for the reactant and products in benzaldehyde amination with nitrobenzene over Co supported on nitrogen doped carbon in the presence of formic acid at 150°C in THF as a solvent, adapted from.[Citation56] Notation: Molar percentage of nitrobenzene (■), N-benzylideneaniline (●), aniline (▲) and N-benzylaniline (o). Copyright permission from Elsevier Ltd.
![Figure 17. Concentration profiles for the reactant and products in benzaldehyde amination with nitrobenzene over Co supported on nitrogen doped carbon in the presence of formic acid at 150°C in THF as a solvent, adapted from.[Citation56] Notation: Molar percentage of nitrobenzene (■), N-benzylideneaniline (●), aniline (▲) and N-benzylaniline (o). Copyright permission from Elsevier Ltd.](/cms/asset/82e13666-08ce-4ec1-b728-b24d9f284861/lctr_a_1942689_f0017_b.gif)
Figure 18. Effect of CO pressure in reductive amination of benzaldehyde with a) nitrobenzene over Co supported on nitrogen modified carbon catalyst in water at 170°C after 10 h adapted from[95] notation: solid symbol – amine, open symbol – imine and b) 4-methoxynitrobenzene over Co2Rh2/C at 120°C after 12 h in THF and a small amount of water adapted from,[Citation54] notation: solid symbol – amine, open symbol – tertiary amine, a and b denote the yield of secondary amine and tertiary amine, respectively after 24 h. Copyright permissions from Elsevier Ltd and from American Chemical Society.
![Figure 18. Effect of CO pressure in reductive amination of benzaldehyde with a) nitrobenzene over Co supported on nitrogen modified carbon catalyst in water at 170°C after 10 h adapted from[95] notation: solid symbol – amine, open symbol – imine and b) 4-methoxynitrobenzene over Co2Rh2/C at 120°C after 12 h in THF and a small amount of water adapted from,[Citation54] notation: solid symbol – amine, open symbol – tertiary amine, a and b denote the yield of secondary amine and tertiary amine, respectively after 24 h. Copyright permissions from Elsevier Ltd and from American Chemical Society.](/cms/asset/721965b6-1526-4016-aa11-0012862b2d79/lctr_a_1942689_f0018_b.gif)
