Figures & data
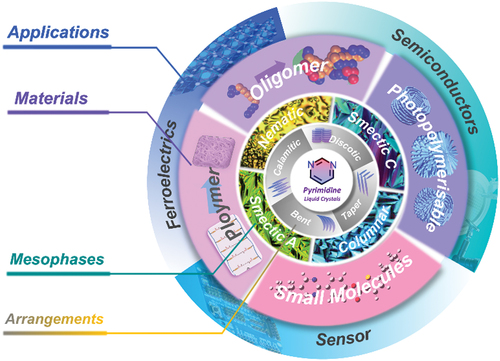
Figure 2. (Colour online) The schematic exhibition of structures and mesophases for pyrimidine liquid crystals based on geometric shape, flexible chains, molecular polarity and polarizability, etc.
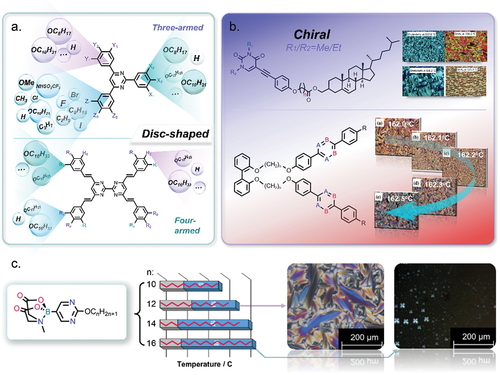
Figure 3. (Colour online) The chemical structures, mesophase transition temperatures and POM textures of boron pyrimidine liquid crystals [Citation11].
![Figure 3. (Colour online) The chemical structures, mesophase transition temperatures and POM textures of boron pyrimidine liquid crystals [Citation11].](/cms/asset/9c223362-e3eb-46aa-a024-5ee9466f19d3/tlct_a_2361294_f0003_oc.jpg)
Figure 4. (Colour online) The chemical structures, mesophase transition temperatures and POM textures of cholesteric liquid crystals.
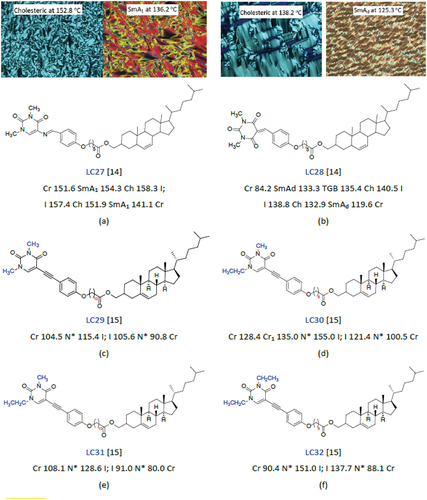
Table 1. The chemical structures and mesophase transition temperatures (oC) of nitrogen-substituted 5CB derivatives.
Table 2. The chemical structures and mesophase transition temperatures (oC) of discotic pyrimidine liquid crystals [Citation12,Citation13].
Figure 5. (Colour online) The chemical structures, mesophase transition temperatures and POM textures of laterally connected pyrimidine liquid crystals [Citation16].
![Figure 5. (Colour online) The chemical structures, mesophase transition temperatures and POM textures of laterally connected pyrimidine liquid crystals [Citation16].](/cms/asset/66860b88-df3c-45c0-b0fe-92b0e9fc7bde/tlct_a_2361294_f0005_oc.jpg)
Table 3. The chemical structures and mesophase transition temperatures (oC) of bipyrimidine discotic liquid crystals.
Figure 6. (Colour online) The schematic exhibition of pyrimidine-based liquid crystalline structures and mesophases for ferroelectric application.
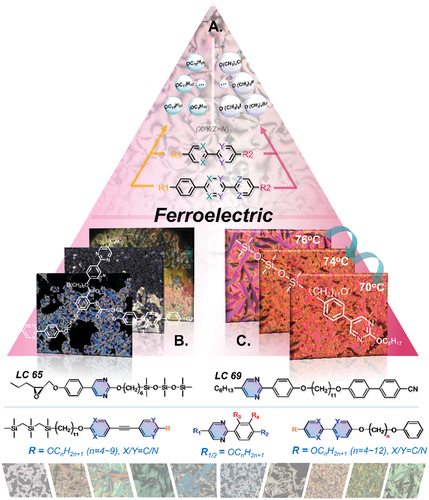
Table 4. The chemical structures and mesophase transition temperatures (oC) of phenyl pyrimidine liquid crystals.
Table 5. The chemical structures and mesophase transition temperatures (oC) of pyrimidine-based liquid crystals with epoxy [Citation79], branched tricarbosilane [Citation82], bent-core dimers [Citation83], taper-shaped trimers [Citation84].
Table 6. The chemical structures and mesophase transition temperatures (oC) of biphenyl pyrimidine compounds with siloxane/carbosilane terminal chains.
Figure 7. (Colour online) The chemical structures, mesophase transition temperatures (oC) and POM textures of 2(n) and 3(n) series of liquid crystals [Citation85].
![Figure 7. (Colour online) The chemical structures, mesophase transition temperatures (oC) and POM textures of 2(n) and 3(n) series of liquid crystals [Citation85].](/cms/asset/791d06bd-99f8-4504-83b5-9750798dc551/tlct_a_2361294_f0007_oc.jpg)
Table 7. The chemical structures and mesophase transition temperatures (oC) of phenyl-pyrimidine liquid crystals with siloxane/carbosilane terminal chains.
Table 8. Effect of halogen end-groups with fluoro-, bromo-, iodo-substitution on mesophases and their transition temperatures for phenyl-pyrimidine liquid crystals.
Table 9. The chemical structures and mesophase transition temperatures (oC) of tricarbosilane-terminated phenylpyrimidine with ethynyl spacer.
Table 10. The chemical structures and mesophase transition temperatures (oC) of pyrimidine-based liquid crystals with phenoxy end groups.
Table 11. The chemical structures and mesophase transition temperatures (oC) of thienyl-phenyl-pyrimidine compounds.
Table 12. The chemical structures and mesophase transition temperatures (oC) of thienyl-pyrimidine liquid crystals.
Table 13. The chemical structures and mesophase transition temperatures (oC) of phenyl pyrimidine liquid crystals with chiral centres.
Figure 8. (Colour online) The schematic exhibition of pyrimidine-based polycatenar oligomers, photopolymerisable liquid crystals and copolymers for application in organic semiconductors.
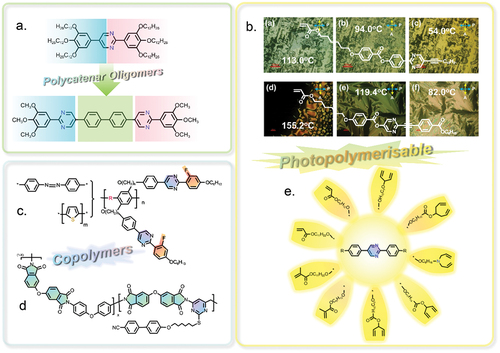
Table 14. The chemical structures of pyrimidine-based copolymers.
Figure 9. (Colour online) The chemical structures, mesophase transition temperatures (oC) and POM textures of photopolymerisable pyrimidine liquid crystals [Citation145].
![Figure 9. (Colour online) The chemical structures, mesophase transition temperatures (oC) and POM textures of photopolymerisable pyrimidine liquid crystals [Citation145].](/cms/asset/7eafe79e-17d4-4ffa-b5b8-2694ece4e322/tlct_a_2361294_f0009_oc.jpg)
Table 15. The chemical structures and mesophase transition temperatures (oC) of azobenzene pyrimidine compounds.
Table 16. The chemical structures and mesophase transition temperatures (oC) of pyrimidine-based photopolymerisable liquid crystals.
Table 17. The chemical structures and mesophase transition temperatures (oC) of pyrimidine-based polycatenary oligomers.
Figure 10. (Colour online) The schematic exhibition of pyrimidine-based liquid crystals coordinated with palladium and platinum as metallomesogens and non-conventional functional mesomorphic materials for application in sensors.
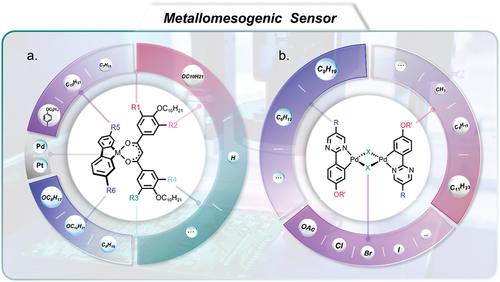
Table 18. The chemical structures and mesophase transition temperatures (oC) of pyrimidine-based organometallic calamitic/discotic liquid crystals.
Table 19. The chemical structures and mesophase transition temperatures (oC) of binuclear cyclopalladated phenylpyrimidine compounds.