Figures & data
FIG. 1. Experimentally determined stoichiometric osmotic coefficients (φst) plotted against the square root of the total sulfate molality (mSO42−), for aqueous mixtures of two aminium sulfates with H2SO4, at three different aminium:sulfate molar ratios (noted on the plots). Different symbols are used for each ratio. The solid line shows values for aqueous (NH4)2SO4 (Clegg et al. Citation1995), and the dashed line aqueous H2SO4 (Clegg and Brimblecombe Citation1995). (a) Methylaminium sulfate (MAS). (b) Dimethylaminium sulfate (DMAS). Uncertainties in φst resulting from the H2SO4 titration, as well as the IC and aw Measurements as propagated through the calculations, are displayed on the vertical dimension unless they are smaller than the symbols.
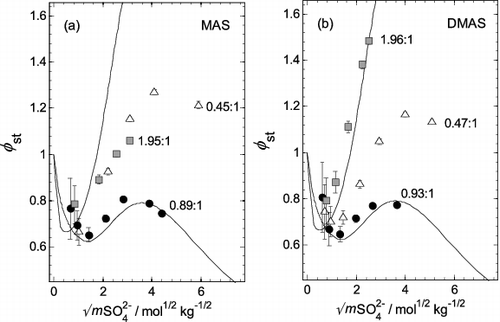
FIG. 2. Aminium sulfate (msalt) and H2SO4 molalities (macid) in the mixtures, interpolated for fixed water activities from 0.65 to 0.95 (indicated on the plots). Symbols: values from Table S2. Lines: calculated using the fitted extended ZSR model (Equation (3)) using coefficients from Table 1. (a) MAS. (b) EAS. (c) DMAS. (d) DEAS. (e) TMAS. (f) Molalities of aqueous (NH4)2SO4–H2SO4 mixtures, calculated using the model of Clegg et al. (Citation1998).
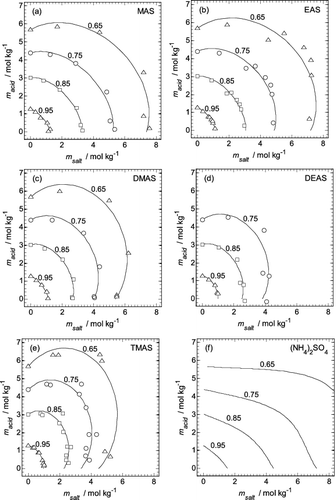
FIG. 3. The mass (kg) of water per mole of total solutes, at a water activity of 0.70, plotted against the dry mole fraction of aminium sulfate salt present (xsalt), from pure aqueous H2SO4 (xsalt = 0.0) to pure aqueous aminium sulfate (xsalt = 1.0). Symbols: open circle—interpolated values from Table S2; filled circle—pure aqueous H2SO4 from the work of Clegg and Brimblecombe (Citation1995). Lines: calculated using the fitted extended ZSR model (Equation (3)) using coefficients from Table 1. (a) MAS. (b) EAS. (c) DMAS. (d) DEAS. (e) TMAS. Uncertainties are not shown because they are smaller than the sizes of the plotted symbols.
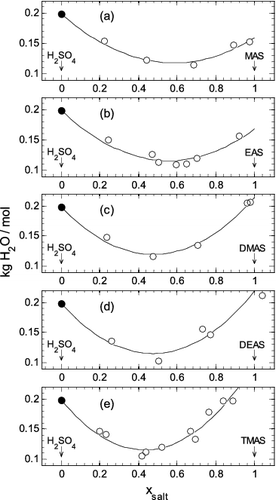
FIG. 4. Stoichiometric osmotic coefficients (φst) plotted against the square root of the total sulfate molality (mSO42−), for aqueous mixtures of all five aminium sulfates with H2SO4, at two different aminium:sulfate molar ratios (noted on the plots). Symbols: solid and shaded—experimental values and uncertainties (Table S1); open symbols—interpolated (Table S2). Lines: solid—from the fit of the extended ZSR model (Equation (3)), with coefficients listed in Table 1; dashed—these indicate the effects of the uncertainties in the fit of Equation (3). (a) MAS. (b) EAS. (c) DMAS. (d) DEAS. (e) TMAS. (f) Additional results for EAS and TMAS (as indicated) for ratios close to those shown in plots (b) and (e).
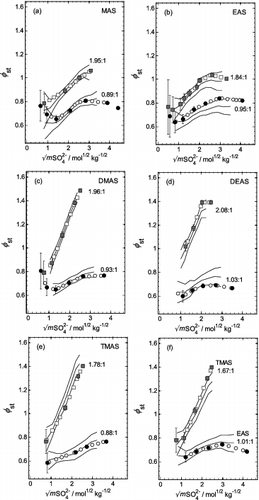
FIG. 5. Stoichiometric osmotic coefficients (φst) of aminium bisulfates (aminium:sulfate ratio 1:1) and aminium sulfates (ratio 2:1) plotted against the square root of total sulfate molality (mSO42−). Symbols: values, with uncertainties, calculated using the extended ZSR model (Equation (3)) with parameters listed in Table 1. Lines: values for aqueous NH4HSO4 (plot a) and (NH4)2SO4 (plot b) calculated from the results of Clegg et al. (Citation1998), and Clegg et al. (Citation1995), respectively. The identities of the aminium salts in the mixtures are indicated on the plots. (a) Aqueous aminium bisulfates, and NH4HSO4. For clarity, the results for sulfates EAS to TMAS are offset vertically by the amounts indicated in parentheses on the plot. (b) Aqueous aminium sulfates, and (NH4)2SO4.
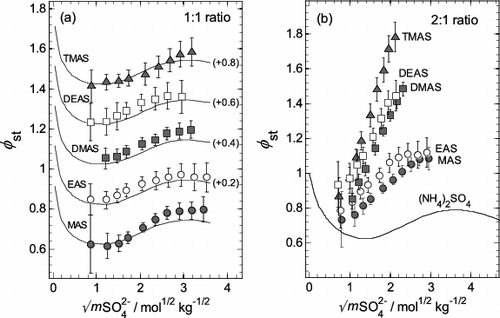
FIG. 6. Comparison of water uptake by different aminium salts plotted as moles of water (nH2O) per mole of salt against equilibrium relative humidity (RH, as a fraction). Symbols: circle—methylaminium; solid circle—ethylaminium; square—dimethylaminium; solid square—diethylaminium; triangle—trimethylaminium. (a) Sulfates (this study). Values for the points were calculated using the extended ZSR model (Equation (3)), with coefficients in Table 1. The lowest line on the plot is for aqueous (NH4)2SO4 and was calculated using the results of Clegg et al. (Citation1995). Other lines, for each aminium sulfate, were calculated using Equation (4) and the parameters in Table 2. (b) Sulfates (Clegg et al. Citation2013). The values for the points were taken from their Table 6. The line is for aqueous (NH4)2SO4, the same as in (a). (c) Nitrates (Bonner Citation1981). The line is for aqueous NH4NO3, calculated using the results of Clegg et al. (Citation1998). (d) Chlorides (Macaskill and Bates Citation1986). The line is for aqueous NH4Cl, calculated using the equation of Liang and Chan (Citation1997) and results of Myerson et al. (Citation1996).
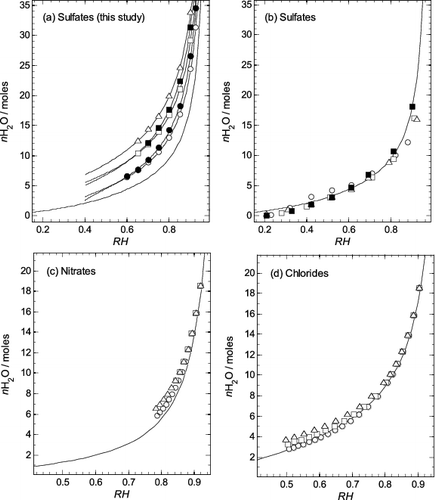
FIG. 7. Stoichiometric osmotic coefficients (φst) of the aqueous aminium sulfates, plotted against the square root of sulfate molality (mSO42−). Symbols: osmotic coefficients of each salt (as indicated on the plot) calculated using Equation (3) with the coefficients in Table 1. Lines: osmotic coefficients fitted using the ion interaction model (Equation (4)), and calculated using the parameters in Table 2. The uncertainties associated with the data points are omitted from this plot, but can be seen in Figure 5b.
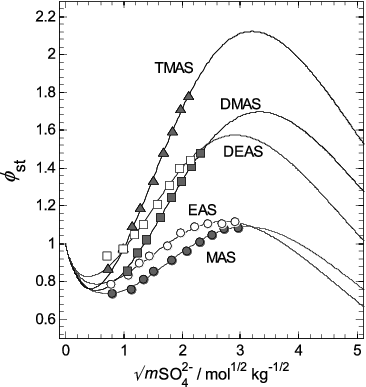
TABLE 1 Parameters of the extended ZSR model (Equation (3)), at fixed water activities (aw), for mixtures of aminium sulfate salts and H2SO4
TABLE 2 Fitted parameters of the ion interaction model (Equation (4)) for the aminium sulfate saltsa,b
