Figures & data
Table 1. SEM images showing surface morphology, including crystal size, of the dried microparticles.
Figure 1. The particle density as a function of time during evaporation and particle formation for droplets with equal molar amounts of NaNO3 and KNO3. The full symbol curves were calculated assuming no voids in the particle, i.e., a non-crystallizing system. The open symbol curves were calculated using the final diameter of the actual dry particles. The larger data point approximates the onset of shell formation.
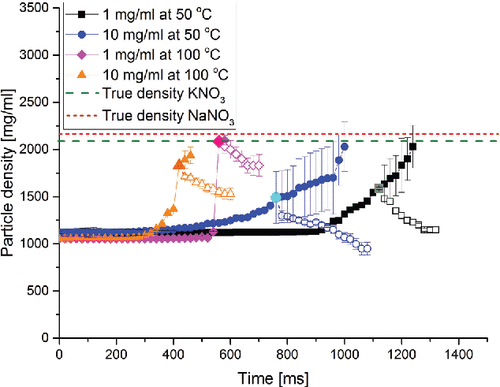
Table 2. Helium ion microscope images of sectioned microparticles dried from droplets with an initial concentration of 10 mg/ml.
Table 3. Low frequency shift Raman spectra of dried microparticles compared with the reference spectra of crystalline NaNO3 and crystalline KNO3. Cases with an initial concentration of 10 mg/ml are shown. Molar percentages are shown as a reference.
Figure 2. Particle density of the final dried microparticles as a function of the precipitation window for the component that first saturates on the surface. The solid lines at the top indicate the true densities of KNO3 and NaNO3.
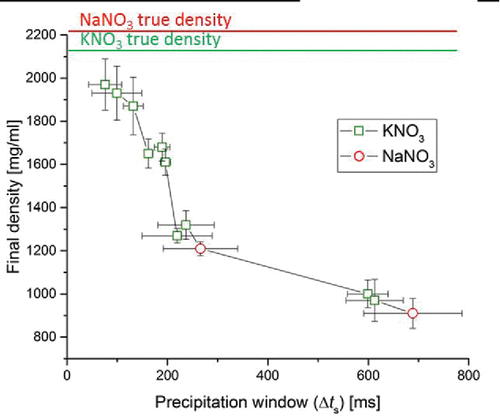
Figure 3. Surface concentrations of NaNO3 and KNO3 in evaporating droplets as a function of time for the cases with an initial concentration of 1 mg/ml at 50°C drying gas temperature and molar percentages of NaNO3 of 30% or 70% or weight percentages of NaNO3 of 26.5% or 66.2%. ts indicates the time to reach saturation. On the right-hand side, the plot of surface saturation normalized by the solubility of each solute is shown. The time to reach saturation is obtained when the surface concentration equals the solubility. The time for crystallization is reached at a supersaturation level of around 1.6 for NaNO3 and 1.9 for KNO3.
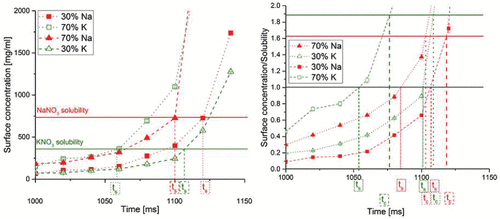
Table 4. SEM/EDX maps of the chemical components near the surface of the dried particles. Below each map the measured molar ratio of NaNO3 to KNO3 is listed together with the time differences between saturation and crystallization events for the components. Positive values indicate earlier saturation and crystallization for KNO3.
Table 5. Elemental composition across the shell of sectioned microparticles determined by S-TEM. Peaks shifted to the left represent the chemical components present on the surface. Cases with a solution concentration of 10 mg/ml dried at 50°C for molar percentages of 70% of NaNO3 and 30% of KNO3 and 50% of NaNO3 and 50% of KNO3 are shown.
