Figures & data
Figure 1. Simple representation of the autoxidation mechanism that leads to HOM formation from an alkylperoxy radical (Crounse et al. Citation2013). The resulting HOM in this scheme is a hydroperoxycarbonyl, like the AHPA model compound studied here.
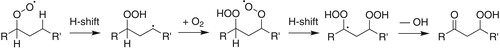
Figure 2. Mechanism of reaction of cyclodecene with O3 in the presence of 1-propanol to form 10-n-propoxy-10-hydroperoxy decanal (AHPA).
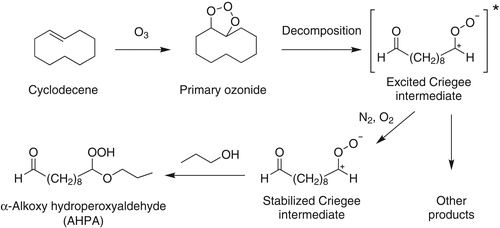
Figure 3. Potential reactions of AHPA in SOA. In the concerted elimination and strong acid decomposition pathways H2, water, and propanol co-products are not shown.
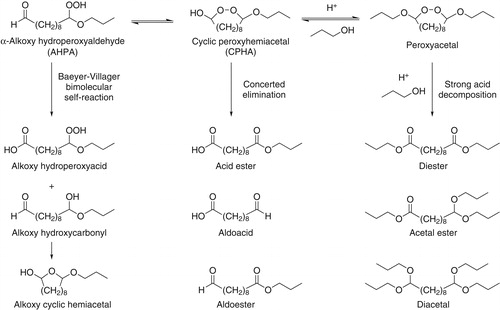
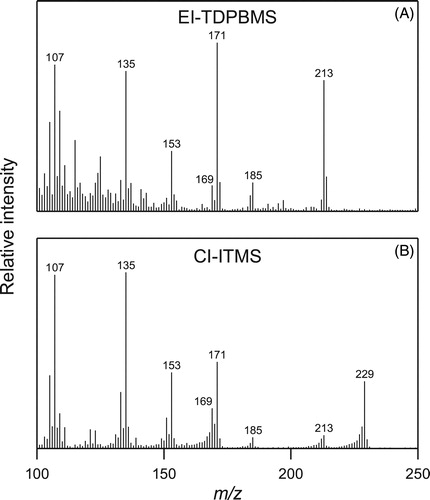
Table 1. Characteristic ions and fragmentation pathways for major products in SOA analyzed by EI-TDPBMS and CI-ITMS.
Figure 5. Time profile of the ratio of real-time EI-TDPBMS mass spectral signals measured at peaks characteristic of CPHA (m/z 169) and AHPA (m/z 171) in SOA formed from reaction of cyclodecene with O3 in the presence of 1-propanol and DOS seed particles. The data were fit to an exponential function to determine the timescale to establish cyclization equilibrium (τeq) and the equilibrium constant (Keq).
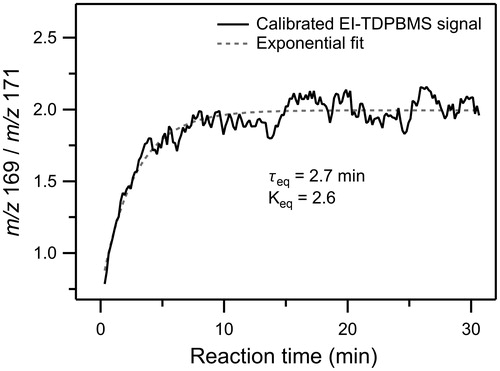
Figure 6. Time profiles of peroxide functional group content of SOA formed from the reaction of cyclodecene with O3 in the presence of 1-propanol and seed particles with the following composition: (squares) aqueous ammonium sulfate/sulfuric acid at 50% RH and pH = 1, (circles) dry DOS, and (diamonds) dry DOS/sulfuric acid.
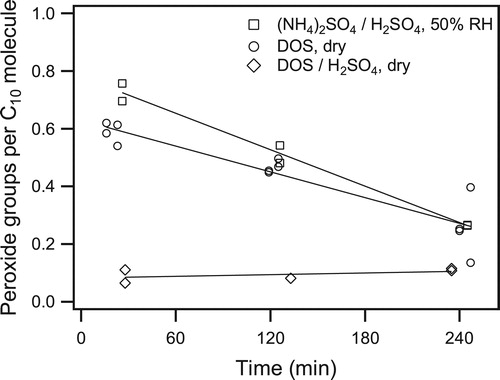
Figure 7. Real-time EI-TDPBMS mass spectra measured at different times for SOA formed from the reaction of cyclodecene with O3 in the presence of 1-propanol and seed particles with different compositions as follows: (a) immediately after formation on dry DOS, (b) 4 h after formation on dry DOS, (c) immediately after formation on dry DOS/sulfuric acid, and (d) 4 h after formation on aqueous ammonium sulfate/sulfuric acid at 50% RH and pH = 1.
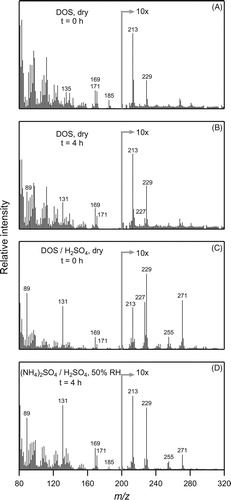
Figure 8. CI-ITMS mass spectra of filter extracts of SOA collected at different times from the reaction of cyclodecene with O3 in the presence of 1-propanol in the absence or presence of seed particles as follows: (a) immediately after formation in the absence of seed particles, (b) 4 h after formation in the absence of seed particles, and (c) immediately after formation on dry sulfuric acid seed particles. DOS seed particles were not used in these experiments because DOS is the dominant signal in CI-ITMS at the concentrations used in chamber experiments.
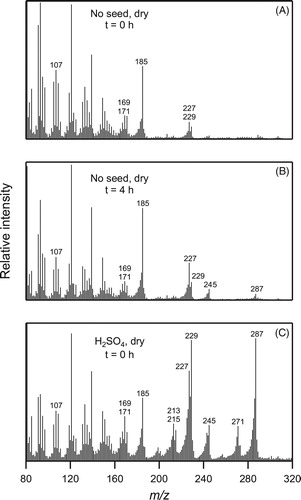
Table 2. Molar yields of functional groups in SOA formed in the presence of CO without seed particles or 1-propanol with sulfuric acid seed particles.
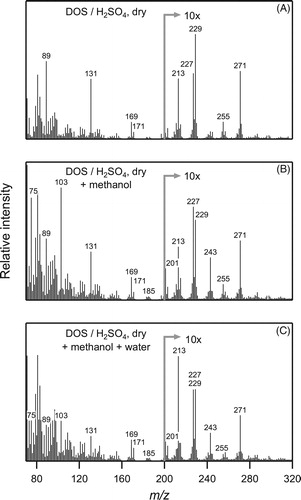
Figure 9. Real-time EI-TDPBMS mass spectra of (a) SOA formed from the reaction of cyclodecene with O3 in the presence of 1-propanol and dry DOS/sulfuric acid seed particles, and with the subsequent addition of (b) methanol, and then (c) water.