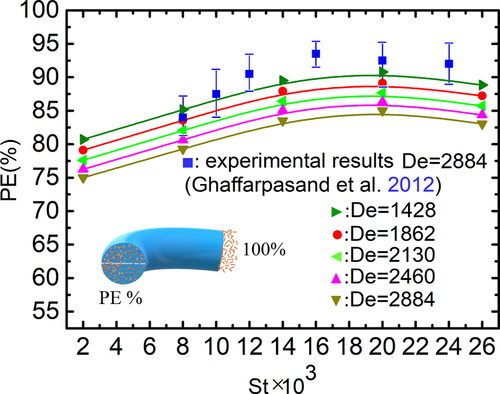
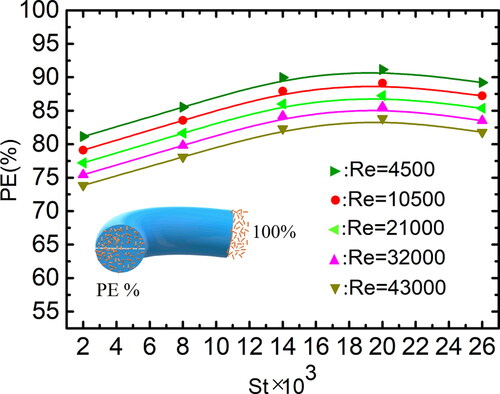
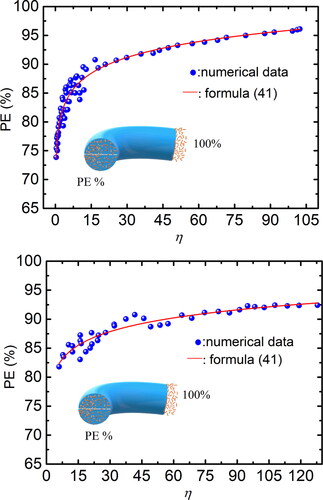
Figures & data
Figure 4. Distribution of mean axial velocity (a) and RMS value of fluctuating axial velocity (b) along the tube midplane (white line) (Re = 10,500, De = 2460).
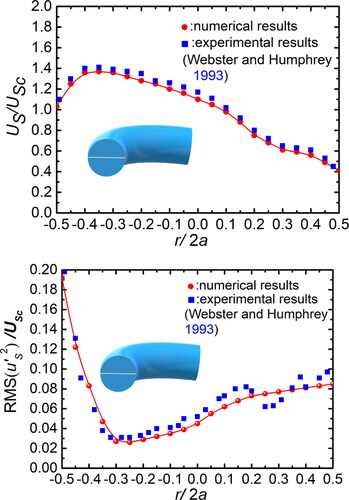
Figure 5. Distribution of relative particle number concentration on the cross-section at different axial positions (Re = 10,500, St = 0.014, De = 1862, β = 8). (a) S/Lb =0, (b) S/Lb =1/3, (c) S/Lb =2/3, and (d) S/Lb = 1.
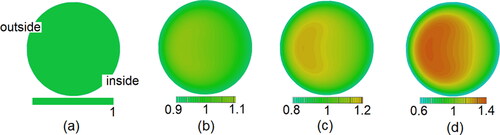
Figure 6. Distributions of mean orientation of particles on the cross-section at different axial positions. (Re = 10,500, St = 0.014, De = 1862, β = 8).
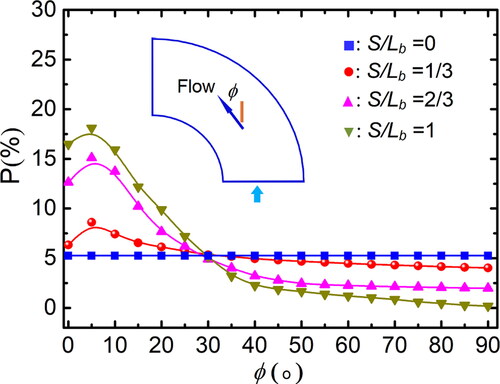
Figure 7. Distribution of relative particle number concentration on the cross-section at outlet. (a) St = 0.020, De = 1862, Re = 10,500, β = 8, (b) St = 0.014, De = 2460, Re = 10,500, β = 8, (c) St = 0.014, De = 1862, Re = 4500, β = 8, and (d) St = 0.014, De = 1862, Re = 10,500, β = 4.
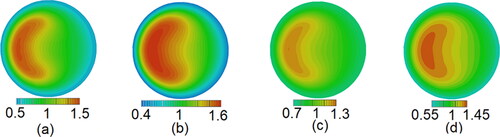
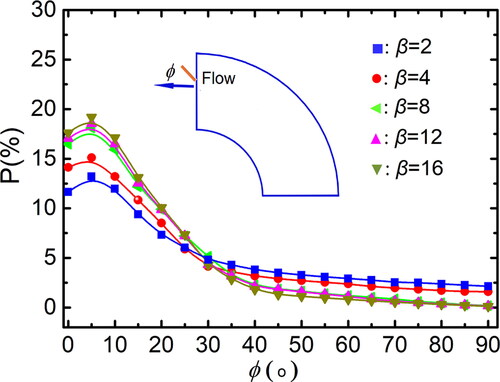
Figure 11. Distribution of particle orientation for different aspect ratio (Re = 10,500, De = 1862, St = 0.014).