Figures & data
Figure 1. Creating genetically modified chickens. A) Transposon plasmids or genome editing tools are directly injected into the laid egg or the circulatory system of day 2.5 embryos. The embryos are hatched, raised to sexual maturity, and bred. Breeding these F0 chickens will produce F1 offspring containing the transposon or genetic modification. B) PGCs are cultivated from blood samples from day 2.5 chicken embryos and subsequently transformed with a transposon vector or genome editing tools. These transfected cultures are sorted and grown clonally to produce transgenic PGC cultures containing the desired modification. The genetically modified PGCs are then injected into day 2.5 host embryos and then hatched to create mature F0 chickens with some modified germ cells. Crossing these chickens with wildtype chickens produces several F1 offspring that will be derived from the donor germ cells.
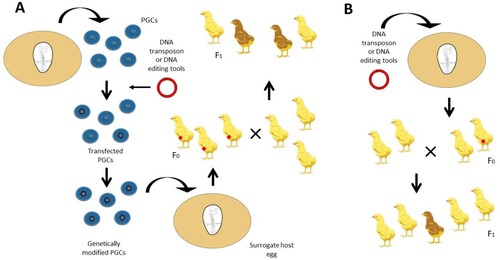
Figure 2. In vitro cell systems to study avian pathogens. Cell lines, primary cells, and pluripotent stem cells are all useful for studying host-pathogen interaction in avian species. Each system has its advantages and disadvantages.
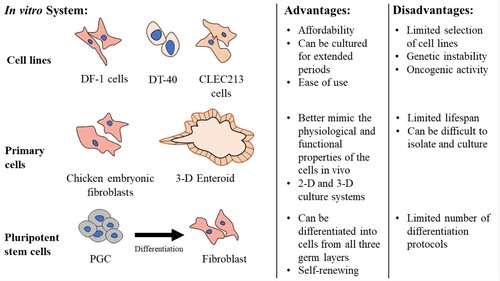
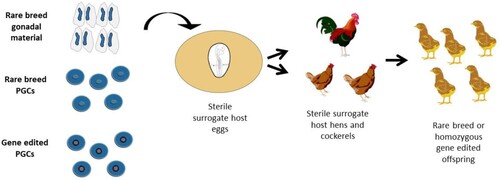
Figure 3. Generating homozygous genome-edited or rare breed offspring using sterile surrogate hosts. Embryonic gonads, cultured PGCs, or gene-edited PGCs can be cryopreserved before injection into sterile surrogate host embryos. The surrogate hosts are hatched and raised to sexual maturity. When mated, the offspring are entirely derived from the donor genetic material.