Figures & data
Table 1. Diameter and wall thickness of oospores from several isolates of Phytophthora capsici.
Fig. 1 (Colour online) Development of P. capsici on greenhouse vegetables at various stages following inoculation. (a) Healthy roots and stem of greenhouse ‘Cubico’ pepper. (b) Early stage dark brown discolouration of roots (arrow) and dark stem canker formation (arrow) on greenhouse ‘Cubico’ pepper 1 week after inoculation (c) Progressive wilting of infected greenhouse ‘Cubico’ pepper plants (arrows). (d) Internal brown–black discolouration within the stem and crown tissue of infected greenhouse ‘Cubico’ pepper. The central part of the stem is also hollow and dried. (e) Severe symptoms on greenhouse ‘Cubico’ pepper 2 months after inoculation. The leaves are necrotic, wilted and yellowish–brown in colour (arrow). The stem and branches are very stiff and hollow, and yellowish-green colour. The plant is dried-up, the fruits are shrivelled and partially rotted. (f) Stem and fruit rot on infected greenhouse ‘Cubico’ pepper plant. The pepper plant was inoculated on the fruit and at the branches. The leaves have wilted and the fruit is decayed and shrivelled. Some white mycelial growth due to P. capsici is visible on the top part of the fruit and on the stem (arrows). (g) Foliar wilt of greenhouse ‘Corona’ cucumber 7 days after inoculation. (h) Decayed stem of infected greenhouse ‘Corona’ cucumber plant. The lower stem is yellowish-green colour and is shrivelled (arrow) compared with the upper healthy part. (i) Later stage of stem canker formation on ‘Corona’ cucumber. Stem canker is yellow-brown (arrow) with oldest leaves now necrotic (not shown). (j) Foliar wilt and necrosis of ‘Corona’ cucumber (arrow) during the late stage of infection. (k) Internal dark brown discolouration of vascular stem tissue (arrow) and dark brown roots during the late stage of infection of ‘Corona’ cucumber. (l) Foliar wilt of greenhouse ‘Trust’ tomato 12 days after inoculation. (m) Initial yellow-brown canker formation (arrow) at the base of infected greenhouse ‘Trust’ tomato and chlorosis of bottom branches (arrow). (n) Dark canker formation (arrow) at stem base during later stage of infection on greenhouse ‘Trust’ tomato. (o) Internal dark brown discolouration of vascular stem tissue (arrow) of infected greenhouse ‘Trust’ tomato.

Fig. 2 Gel electrophoresis of PCR amplified field isolates DT-6B, DT1B-C, DT1D-A, DT1A-A and known P. capsici isolates ATCC-15399 and ATCC-15427. DNA extracts of individual isolates were PCR amplified using PC-1 (5ʹ GTCTTGTACCCTATCATGGCG 3ʹ) and PC-2 (5ʹ CGCCACAGCAGGAAAAGCATT 3ʹ) primers. PCR amplicons were run on a 1% agarose gel containing 10 µL of Invitrogen SYBR safe DNA gel stain. Putative P. capsici field isolates in lanes 3, 4, 6 and 7 as well as the known P. capsici isolates in lanes 5 and 6 displayed the expected 560 bp band (Zhang et al. Citation2006), confirming their identity as P. capsici.
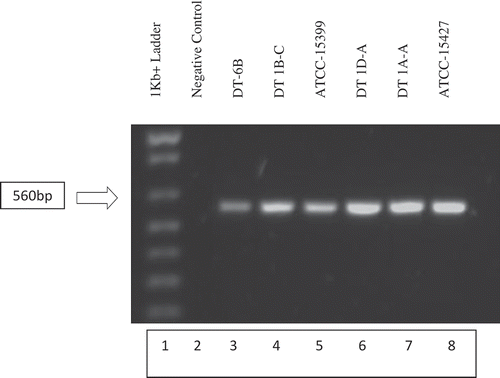
Table 2. Effect of seed treatments on per cent germination (G) and per cent stand (S) of ‘Dark Green’ zucchini in greenhouse tests using soil sampled from three commercial fields near AAFC-GPCRC in 2005 where Phytophthora capsici was previously reported in 2004.
Fig. 3 (Colour online) Efficacy of fungicides for control of Phytophthora capsici in greenhouse tomato (T-1, 2), cucumber (C-1, 2), and pepper (P-1, 2) trials. Drench treatments consisted of: A-control (water), B-fluazinam (Allegro), C-mandipropamid (Revus), D-cyazofamid (Ranman), E-metalaxyl (Ridomil), F-control (No Treatment applied). Plant, roots and crown tissues of tomato, cucumber and pepper were rated separately using 0 (healthy) to 5 (dead) scale 21 days after inoculation (dai) for T-1, 2, 15 and 17 dai for C-1, 2 respectively, and 20 and 13 dai for P-1, 2 respectively. The vertical lines denote standard error.
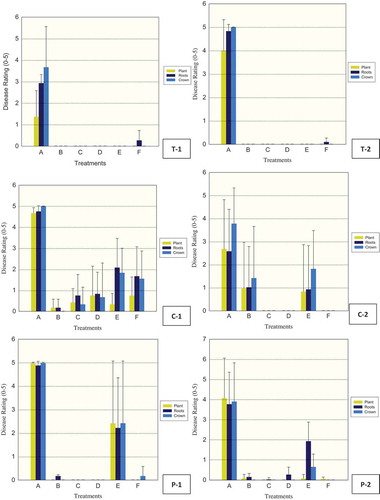
Fig. 4 (Colour online) Efficacy of fungicides for control of Phytophthora capsici in greenhouse pepper (P-3, 4) trials. Drench treatments for P-3 consisted of: A – control (water), B – fluopicolide (Presidio), C, D, E – cyazofamid (Ranman) at 0.15, 0.25 and 0.35 ml L−1, respectively, F – control (No Treatment applied). Drench treatments for P-4 consisted of: A – control (water), B – fluazinam (Allegro), C – mandipropamid (Revus), D, E, F – cyazofamide (Ranman) at 0.15, 0.25 and 0.35 ml L−1, respectively, G – metalaxyl (Ridomil), H – control (No Treatment applied). Plant, roots and crown tissues of pepper were rated separately using 0 (healthy) to 5 (dead) scale 18 and 19 days after inoculation for P-3, 4, respectively. The vertical lines denote standard error.
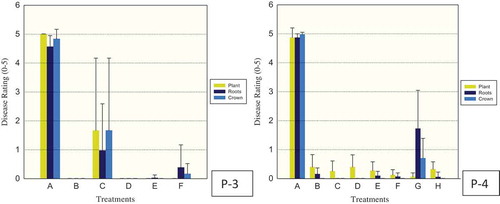
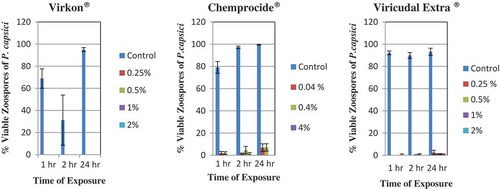
Fig. 5 (Colour online) Effect of hours of contact with various concentrations of disinfectants, Virkon®, Chemprocide® and Virucidal Extra® on per cent germination of zoospores of Phytophthora capsici. Control is sterile distilled water. The vertical lines denote standard error.