Figures & data
Table 1 Inclusion criteria, exclusion criteria, and study controls
Fig. 2 Tetanus IgG Antibody Level. All 3 groups demonstrated an increase in IgG levels at day 45. There was a strongly significant difference between the 1.5 g/day group and the placebo group in IgG levels at day 60 (p = 0.008).
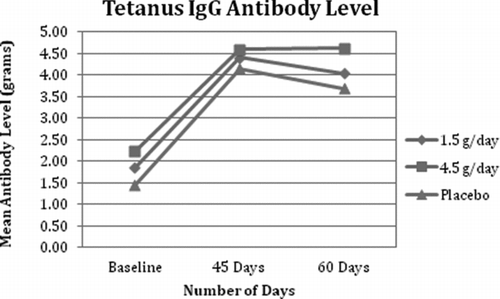
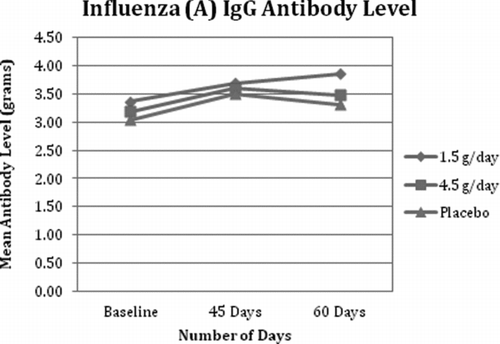
Fig. 3 Influenza (A) IgG Antibody Level. All 3 groups demonstrated an expected physiological increase and peak in influenza A IgM by day 45 with a slight reduction at day 60. All 3 groups demonstrated an expected rise in influenza A IgG following the vaccine, which peaked at day 45 for the 4.5 g/day and placebo groups and at day 60 for the 1.5 g/day group.